Key Documents
I1406
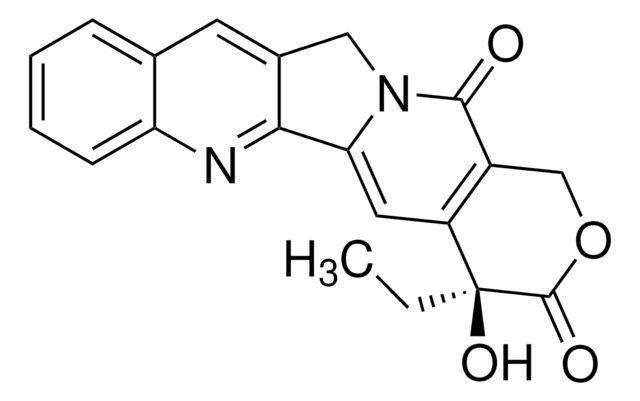
Irinotecan hydrochloride
powder, ≥97% (HPLC)
Synonim(y):
(S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinolin-9-yl ester, CPT-11, [1,4′-Bipiperidine]-1′-carboxylic acid
About This Item
Polecane produkty
product name
Irinotecan hydrochloride, topoisomerase inhibitor
pochodzenie biologiczne
plant (Fructus camptothecae)
Próba
≥97% (HPLC)
Postać
powder
rozpuszczalność
DMSO: 50 mg/mL
temp. przechowywania
2-8°C
ciąg SMILES
Cl.CCc1c2CN3C(=O)C4=C(C=C3c2nc5ccc(OC(=O)N6CCC(CC6)N7CCCCC7)cc15)[C@@](O)(CC)C(=O)OC4
InChI
1S/C33H38N4O6.ClH/c1-3-22-23-16-21(43-32(40)36-14-10-20(11-15-36)35-12-6-5-7-13-35)8-9-27(23)34-29-24(22)18-37-28(29)17-26-25(30(37)38)19-42-31(39)33(26,41)4-2;/h8-9,16-17,20,41H,3-7,10-15,18-19H2,1-2H3;1H/t33-;/m0./s1
Klucz InChI
GURKHSYORGJETM-WAQYZQTGSA-N
informacje o genach
human ... TOP1(7150)
Zastosowanie
- in combination with 5-fluorouracil for screening growth inhibitory functionality in MDA-MB-231 breast cancer cells.
- in chemosensitivity screening of high-grade appendiceal (HGA) and low-grade appendiceal (LGA) organoids.
- as a chemotherapeutic agent in the cytotoxicity studies in combination with heat shock proteins inhibitors (HPSC1) in HT29 colon cancer cells.
Działania biochem./fizjol.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Klasyfikacja zagrożeń
Acute Tox. 4 Oral
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej