Kluczowe dokumenty
T2577
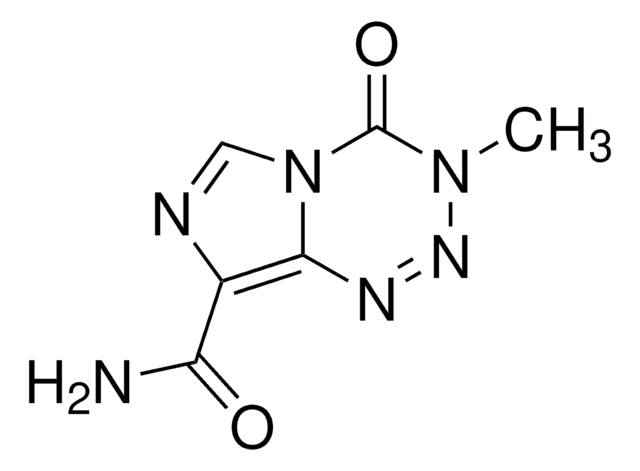
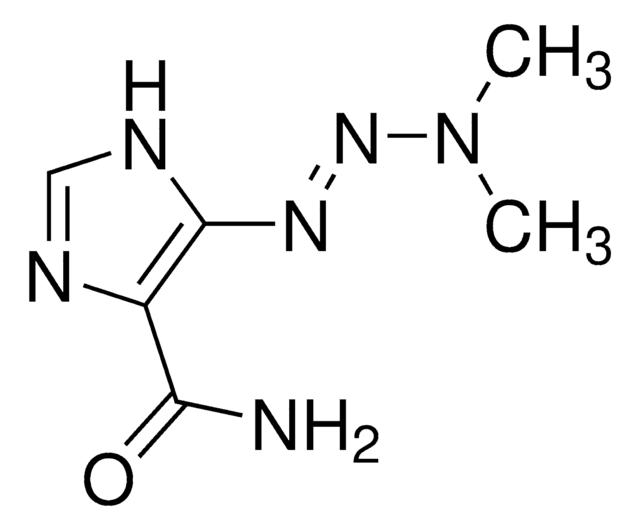
Temozolomide
≥98% (HPLC), powder, DNA methylating agent
Synonim(y):
3,4-Dihydro-3-methyl-4-oxoimidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide, 3-Methyl-4-oxo-8-imidazolo[5,1-d][1,2,3,5]tetrazinecarboxamide, 4-Methyl-5-oxo-2,3,4,6,8-pentazabicyclo[4.3.0]nona-2,7,9-triene-9-carboxamide, 8-Carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one, NSC 362856
About This Item
Polecane produkty
Nazwa produktu
Temozolomide, ≥98% (HPLC)
Poziom jakości
Próba
≥98% (HPLC)
Formularz
powder
kolor
white to light brown
rozpuszczalność
DMSO: 10 mg/mL, clear
H2O: insoluble
inicjator
Schering Plough
temp. przechowywania
2-8°C
ciąg SMILES
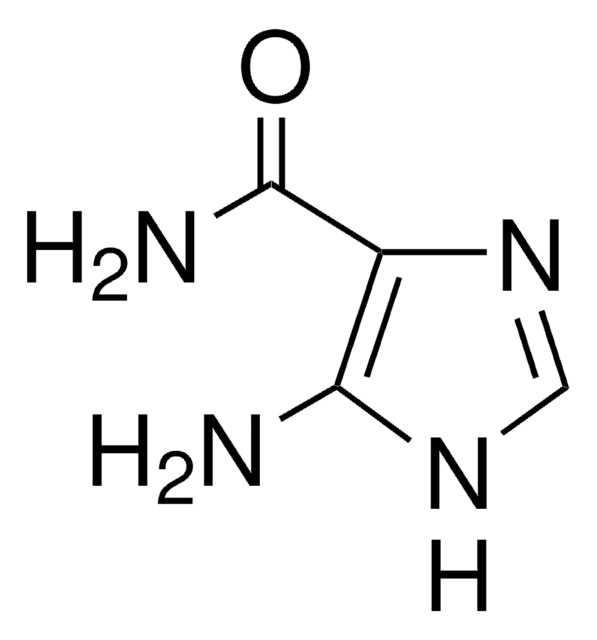
CN1N=Nc2c(ncn2C1=O)C(N)=O
InChI
1S/C6H6N6O2/c1-11-6(14)12-2-8-3(4(7)13)5(12)9-10-11/h2H,1H3,(H2,7,13)
Klucz InChI
BPEGJWRSRHCHSN-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Cechy i korzyści
Uwaga dotycząca przygotowania
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Muta. 1B - Repr. 1B - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
We presents an article on Autophagy in Cancer Promotes Therapeutic Resistance
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej