Kluczowe dokumenty
H6278
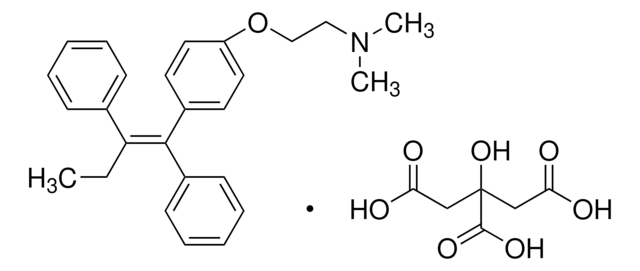
4-Hydroxytamoxifen
≥98% (HPLC), powder, Tamoxifen metabolite
Synonim(y):
4-(1-[4-(Dimethylaminoethoxy)phenyl]-2-phenyl-1-butenyl)phenol, 4-OHT, cis/trans-4-Hydroxytamoxifen
About This Item
Polecane produkty
Nazwa produktu
4-Hydroxytamoxifen, ≥70% Z isomer (remainder primarily E-isomer)
Poziom jakości
Próba
≥98% (HPLC)
Formularz
powder
warunki przechowywania
desiccated
protect from light
rozpuszczalność
methanol: 10 mg/mL
ethanol: 20 mg/mL (with heating)
spektrum działania antybiotyku
neoplastics
Tryb działania
enzyme | inhibits
inicjator
AstraZeneca
temp. przechowywania
2-8°C
ciąg SMILES
CC\C(c1ccccc1)=C(/c2ccc(O)cc2)c3ccc(OCCN(C)C)cc3.CC\C(c4ccccc4)=C(\c5ccc(O)cc5)c6ccc(OCCN(C)C)cc6
InChI
1S/2C26H29NO2/c2*1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h2*5-17,28H,4,18-19H2,1-3H3/b26-25+;26-25-
Klucz InChI
ZJLDABGSDWXVGE-BDSXMVAQSA-N
informacje o genach
human ... ESR1(2099) , ESR2(2100) , ESRRG(2104) , IL6(3569)
rat ... Ar(24208) , Esr1(24890) , Esr2(25149)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
4-OHT effectively inhibited cell growth in the absence of estrogen when cell proliferation was stimulated by insulin or epidermal growth factor. 4-OHT inhibits lipid peroxidation within cell membranes and shows peroxyl radical scavenging activity.
Zastosowanie
- to induce the recombination of small intestinal organoids.
- to study its impact on the ability of human peripheral blood mononuclear cells (PMNCs) to form hematopoietic colonies.
- to induce overexpression of MYCN in the neuroblastoma cell line to determine how the elevated MYCN expression influences the sensitivity of neuroblastoma cells to BIRC5/survivin inhibitor, YM155-induced apoptosis.
Działania biochem./fizjol.
Cechy i korzyści
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
We presents an article on Autophagy in Cancer Promotes Therapeutic Resistance
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej