Key Documents
G1774
Glucagon
≥95% (HPLC), powder, synthetic
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic
Poziom jakości
sterylność
non-sterile
Próba
≥95% (HPLC)
Postać
powder
metody
cell based assay: suitable
rozpuszczalność
1% acetic acid: 1.00-1.04 mg/mL, clear, colorless
water: 1.00-1.04 mg/mL, clear, colorless
przydatność
suitable for molecular biology
numer dostępu UniProt
Warunki transportu
ambient
temp. przechowywania
−20°C
ciąg SMILES
Cl[H].[H]N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](C(C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc4ccc(O)cc4)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc5ccccc5)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc6c[nH]c7ccccc67)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)O)C(O)=O
InChI
1S/C153H225N43O49S.ClH/c1-72(2)52-97(133(226)176-96(47-51-246-11)132(225)184-104(60-115(159)209)143(236)196-123(78(10)203)151(244)245)179-137(230)103(58-83-64-167-89-29-19-18-28-87(83)89)183-131(224)95(43-46-114(158)208)177-148(241)120(74(5)6)194-141(234)101(54-79-24-14-12-15-25-79)182-138(231)105(61-117(211)212)185-130(223)94(42-45-113(157)207)171-124(217)75(7)170-127(220)91(31-22-49-165-152(160)161)172-128(221)92(32-23-50-166-153(162)163)174-146(239)110(69-199)191-140(233)107(63-119(215)216)186-134(227)98(53-73(3)4)178-135(228)99(56-81-33-37-85(204)38-34-81)180-129(222)90(30-20-21-48-154)173-145(238)109(68-198)190-136(229)100(57-82-35-39-86(205)40-36-82)181-139(232)106(62-118(213)214)187-147(240)111(70-200)192-150(243)122(77(9)202)195-142(235)102(55-80-26-16-13-17-27-80)188-149(242)121(76(8)201)193-116(210)66-168-126(219)93(41-44-112(156)206)175-144(237)108(67-197)189-125(218)88(155)59-84-65-164-71-169-84;/h12-19,24-29,33-40,64-65,71-78,88,90-111,120-123,167,197-205H,20-23,30-32,41-63,66-70,154-155H2,1-11H3,(H2,156,206)(H2,157,207)(H2,158,208)(H2,159,209)(H,164,169)(H,168,219)(H,170,220)(H,171,217)(H,172,221)(H,173,238)(H,174,239)(H,175,237)(H,176,226)(H,177,241)(H,178,228)(H,179,230)(H,180,222)(H,181,232)(H,182,231)(H,183,224)(H,184,225)(H,185,223)(H,186,227)(H,187,240)(H,188,242)(H,189,218)(H,190,229)(H,191,233)(H,192,243)(H,193,210)(H,194,234)(H,195,235)(H,196,236)(H,211,212)(H,213,214)(H,215,216)(H,244,245)(H4,160,161,165)(H4,162,163,166);1H/t75-,76+,77+,78+,88-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-;/m0./s1
Klucz InChI
RKGLLHCSSVJTAN-YYICOITRSA-N
informacje o genach
human ... GCG(2641)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
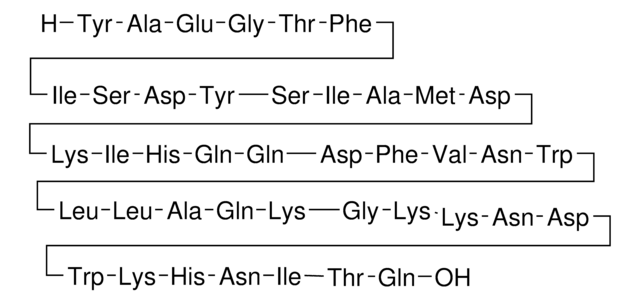
Amino Acid Sequence
Opis ogólny
Zastosowanie
- used as a component of hormone stock solution for preserving full biological activity of heart tissues obtained from male Sprague-Dawley rats
- used as an infusion in phases II and III fasting king penguins to study the various lipolytic, metabolic, and hormonal responses
- used for the stimulation of PGC-1α expression in hepatocytes
- used to induce the expression of methionine adenosyltransferase α1 (MAT1A), which is involved in the regulation of hepatic levels of S-adenosylmethionine and the adaptive response to fasting
- used to study its effect on stimulation of gluconeogenesis through hepatic lipolysis, mediated by inositol trisphosphate receptor 1 (INSP3R1)
- intraperitoneally injected in mice to assess glucagon-induced Sam68 subcellular localization in vivo
Działania biochem./fizjol.
Komponenty
Inne uwagi
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej


![[Arg8]-Vasopressin solution Grade VI (synthetic), ~100 IU/mL in 0.9% NaCl](/deepweb/assets/sigmaaldrich/product/structures/326/242/dede8c26-cf73-4a28-a5d9-1d57c673cf0e/640/dede8c26-cf73-4a28-a5d9-1d57c673cf0e.png)