Key Documents
P0409
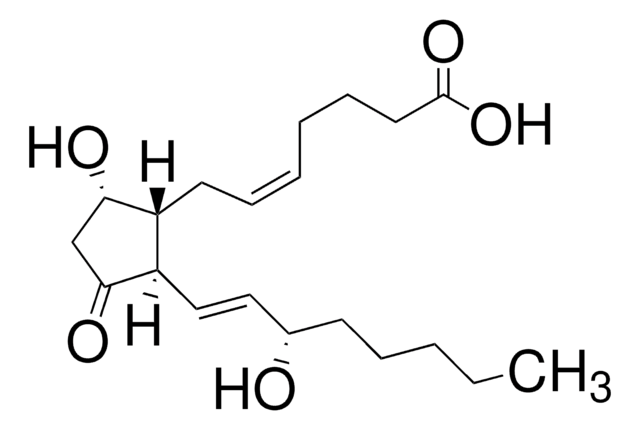
Prostaglandin E2
synthetic, powder, BioReagent, suitable for cell culture
Synonim(y):
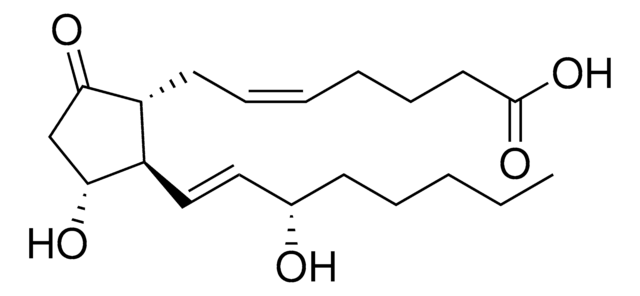
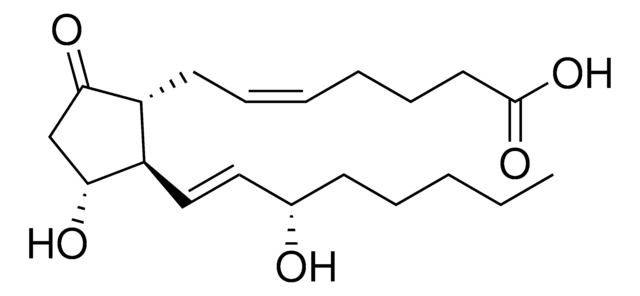
(5Z,11α,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dienoic acid, Dinoprostone, PGE2
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic
Poziom jakości
linia produktu
BioReagent
Próba
≥93% (HPLC)
Postać
powder
siła działania
0.25-100 ng/mL
metody
cell culture | mammalian: suitable
rozpuszczalność
acetone: 10 mg/mL, clear, colorless to faintly yellow
Warunki transportu
ambient
temp. przechowywania
−20°C
ciąg SMILES
O[C@@H]1CC([C@H](C/C=C\CCCC(O)=O)[C@H]1/C=C/[C@@H](O)CCCCC)=O
InChI
1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1
Klucz InChI
XEYBRNLFEZDVAW-ARSRFYASSA-N
informacje o genach
human ... PTGER1(5731) , PTGER2(5732) , PTGER3(5733) , PTGER4(5734) , PTGIR(5739)
mouse ... Ptger1(19216) , Ptger2(19217) , Ptger3(19218) , Ptger4(19219)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Most biologically active prostaglandin. PGE2 induces cervical ripening and parturition; mediates bradykinin-induced vasodilation; regulates adenylyl cyclase. Tumor cells that over-express cyclooxygenase 2 display increased invasiveness, angiogenesis, and resistance to apoptosis that may be due to the PGE2-induced expression of angiogenic factors and stabilization of the anti-apoptotic protein, survivin.
Postać fizyczna
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Repr. 1B
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej