Kluczowe dokumenty
E5389
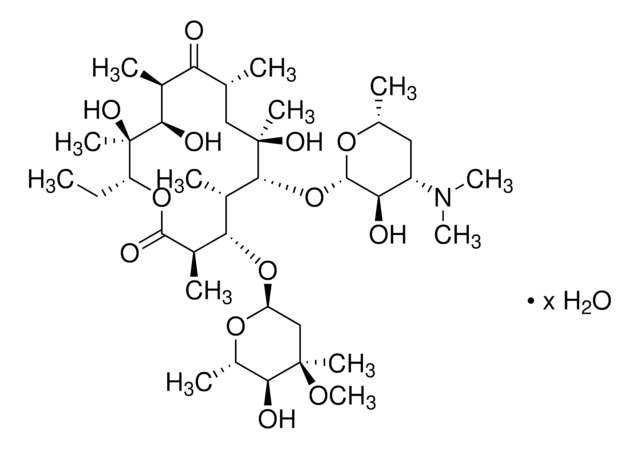
Erythromycin
powder, suitable for cell culture, BioReagent
Synonim(y):
E-Mycin, Erythrocin
About This Item
Polecane produkty
Nazwa produktu
Erythromycin, BioReagent, suitable for cell culture
linia produktu
BioReagent
Poziom jakości
Formularz
powder
siła działania
≥850 μg per mg
metody
cell culture | mammalian: suitable
zanieczyszczenia
≤0.1 EU/mg endotoxin
kolor
white
mp
133 °C
rozpuszczalność
2 M HCl: 50 mg/mL (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
ethanol: soluble (Stock solutions should be stored at 2-8 °C. Stable at 37 °C for 3 days.)
spektrum działania antybiotyku
Gram-negative bacteria
Gram-positive bacteria
Tryb działania
protein synthesis | interferes
ciąg SMILES
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
Klucz InChI
ULGZDMOVFRHVEP-RWJQBGPGSA-N
informacje o genach
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Zastosowanie
- as a supplement for nutrient broth medium for culturing green fluorescent protein (GFP)- expressing E. coli
- as a model drug to determine small intestinal (SMI) microtissue viability using the MTT assay{254
- as an antibiotic to study the treatment strategies of chronic infections
Działania biochem./fizjol.
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Przestroga
Uwaga dotycząca przygotowania
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej