Kluczowe dokumenty
PHR1039
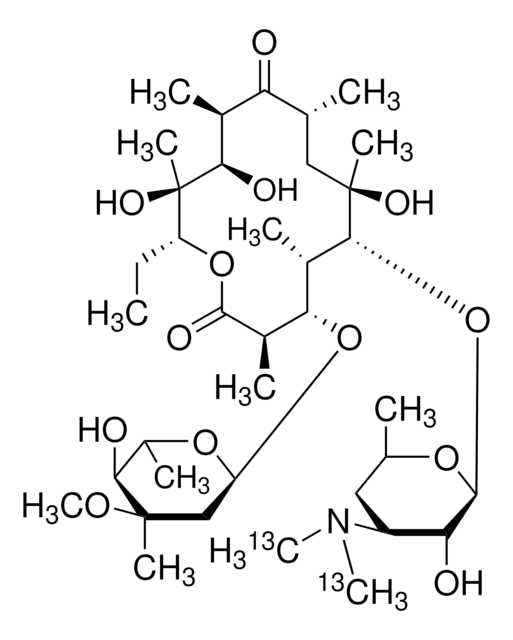
Erythromycin
Pharmaceutical Secondary Standard; Certified Reference Material
About This Item
Polecane produkty
klasa czystości
certified reference material
pharmaceutical secondary standard
Poziom jakości
agency
traceable to BP 794
traceable to Ph. Eur. E1305000
traceable to USP 1242000
rodzina API
erythromycin
Certyfikat analizy
current certificate can be downloaded
metody
HPLC: suitable
gas chromatography (GC): suitable
Zastosowanie
pharmaceutical (small molecule)
Format
neat
temp. przechowywania
-10 to -25°C
ciąg SMILES
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
Klucz InChI
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Erythromycin is a complex macrolide antibiotic drug that exhibits a bacteriostatic activity. It is generally employed in human and veterinary medicines owing to its potential activity against gram positive and a few gram negative strains.
Zastosowanie
Działania biochem./fizjol.
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Przestroga
Uwaga dotycząca przygotowania
Przypis
Informacje prawne
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available. This product was designed, produced and verified for accuracy and stability in accordance with ISO/IEC 17025:2005 (AClass Cert AT-1467), ISO GUIDES 34:2009 (AClass Cert AR-1470).
produkt powiązany
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Analiza porównawcza urządzenia Supel™ BioSPME 96-Pin z techniką szybkiej dializy równowagowej pod kątem dokładności mierzonych wartości, czystości próbki i czasu przepływu pracy w wiązaniu białek leków.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej




![(1S,6R)-6-HYDROXY-5-METHYLENE-1-[(1S)-1,2,3-TRIHYDROXY-2-METHYLPROPYL]-2-OXA-7,9-DIAZABICYCLO[4.2.2]DECANE-8,10-DIONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/271/983/0a99c1ad-6200-4f41-b31f-bd929e66599d/640/0a99c1ad-6200-4f41-b31f-bd929e66599d.png)