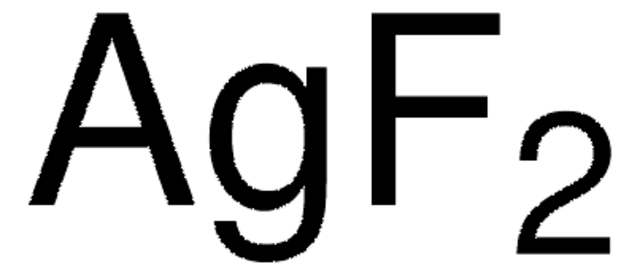Key Documents
ALD00344
PTAD-Azide
95%
Synonim(y):
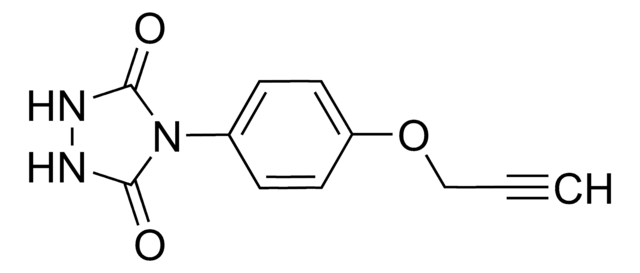
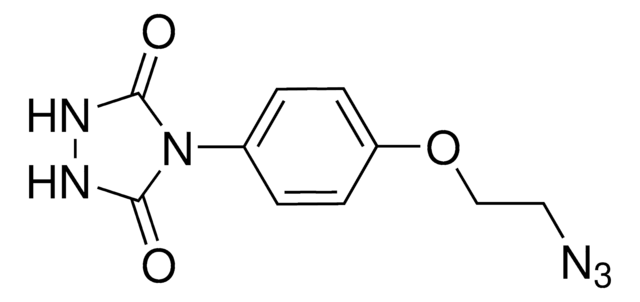
4-(4-(2-Azidoethoxy)phenyl)-1,2,4-triazolidine-3,5-dione, N3-Ph-Ur for e-Y-CLICK
About This Item
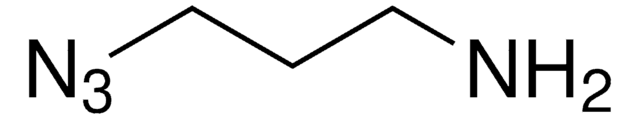
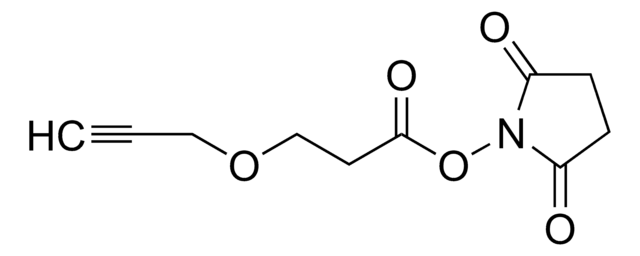
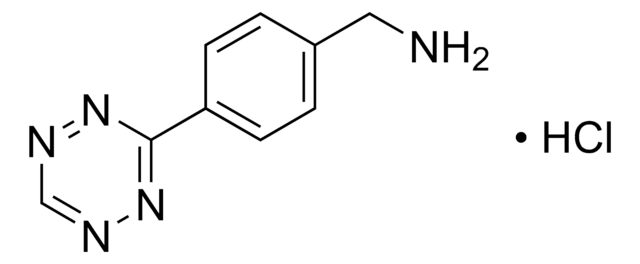
Polecane produkty
Próba
95%
Postać
powder or crystals
przydatność reakcji
reagent type: cross-linking reagent
grupa funkcyjna
azide
temp. przechowywania
2-8°C
ciąg SMILES
O=C(NNC1=O)N1C2=CC=C(OCCN=[N+]=[N-])C=C2
InChI
1S/C10H10N6O3/c11-15-12-5-6-19-8-3-1-7(2-4-8)16-9(17)13-14-10(16)18/h1-4H,5-6H2,(H,13,17)(H,14,18)
Klucz InChI
MHGMHPVYCVQIET-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Note: PTAD-Azide must be first activated by stirring in a 1:0.98 molar ratio with 1,3-dibromo-5,5-dimethylhydantoin (product # 157902). Activation is evident upon solution color change from colorless to deep red and the activated reagent should be used immediately.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
- Mix together 1:0.98 molar equivalents of unactivated PTAD to 1,3-dibromo-5,5-dimethylhydantoin (product # 157902) in organic solvent (preferred solvents are DMF or acetonitrile, avoid using DMSO)
- Color change is observed from colorless/pale yellow to deep red (approximately 5 min of mixing).
- After the solution turns red, store the now activated reagent on ice and use for protein modification within 30 min.
Part 2: Protein modification
- Add protein solution in mixed phosphate/Tris buffer or Tris buffer (pH should be 6 - 9) to the eppendorf tube (or other vial) containing the activated PTAD reagent prepared above and mix gently at room temperature for up to 30 min. Preferably use 10-fold molar excess of reagent relative to protein. Use protein at a minimum concentration of 1 mg/ml (higher concentrations are preferred for enhanced labeling).
- Remove excess unreacted PTAD by gel filtration.
produkt powiązany
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej