Wszystkie zdjęcia(2)
Key Documents
914231
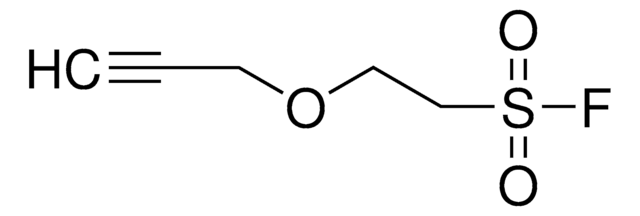
4-(2-(Hex-5-ynamido)ethyl)benzenesulfonyl fluoride
≥95%
Synonim(y):
DAS1, SFABP, Sulfonyl fluoride activity-based probe
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C14H16FNO3S
Numer CAS:
Masa cząsteczkowa:
297.35
Kod UNSPSC:
12352200
NACRES:
NA.22
Polecane produkty
Zastosowanie
4-(2-(Hex-5-ynamido)ethyl)benzenesulfonyl fluoride is an alkyne-functionalized sulfonyl fluoride activity-based probe (SFABP). Sulfonyl fluorides covalently modify reactive serines in addition to threonine, lysine, tyrosine, cysteine, and histidine residues in a context-specific manner. Activity-based probes are useful for the targeting, isolation, and identification of proteins. Furtermore, the alkyne handle on SFABP enables click chemistry-based incorpoation of azide tags, such as fluorophores or biotins, for visualization or enrichment and detection, respectively.
Inne uwagi
Unbiased Mass Spectrometry Elucidation of the Targets and Mechanisms of Activity-Based Probes: A Case Study Involving Sulfonyl Fluorides
Sulfonyl fluorides as privileged warheads in chemical biology
Chemical Proteomics with Sulfonyl Fluoride Probes Reveals Selective Labeling of Functional Tyrosines in Glutathione Transferase
Sulfonyl Fluoride Analogues as Activity-Based Probes for Serine Proteases
Europium-Labeled Activity-Based Probe through Click Chemistry: Absolute Serine Protease Quantification Using 153Eu Isotope Dilution ICP/MS
Sulfonyl fluorides as privileged warheads in chemical biology
Chemical Proteomics with Sulfonyl Fluoride Probes Reveals Selective Labeling of Functional Tyrosines in Glutathione Transferase
Sulfonyl Fluoride Analogues as Activity-Based Probes for Serine Proteases
Europium-Labeled Activity-Based Probe through Click Chemistry: Absolute Serine Protease Quantification Using 153Eu Isotope Dilution ICP/MS
This page may contain text that has been machine translated.
produkt powiązany
Numer produktu
Opis
Cennik
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2
Kod klasy składowania
11 - Combustible Solids
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Lot/Batch Number
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Arjun Narayanan et al.
Chemical science, 6(5), 2650-2659 (2015-05-01)
Sulfonyl fluoride electrophiles have found significant utility as reactive probes in chemical biology and molecular pharmacology. As warheads they possess the right balance of biocompatibility (including aqueous stability) and protein reactivity. Their functionality is privileged in this regard as they
Xiaowen Yan et al.
Angewandte Chemie (International ed. in English), 51(14), 3358-3363 (2012-02-22)
Click and analyze: the titled probe was synthesized by conjugating a sulfonyl fluoride and azido unit using click chemistry to give SF-Eu, which can react specifically with serine (Ser) in the active site of serine protease (SP). Combination of the
Thomas E J Chavas et al.
ACS chemical biology, 13(10), 2897-2907 (2018-09-08)
The elucidation of protein/drug interactions remains a major challenge in drug discovery. Liquid chromatography-tandem mass spectrometry has emerged as a tremendously powerful technology for this endeavor, but its full potential has yet to be realized owing in part to unresolved
D Alexander Shannon et al.
Chembiochem : a European journal of chemical biology, 13(16), 2327-2330 (2012-09-26)
Enriched with fluoride: To expand on the available tools to interrogate proteases, we explored sulfonyl fluorides as activity-based probes. An alkyne-tagged sulfonyl fluoride covalently modifies members of the S1 family of serine proteases. By applying click chemistry, avidin enrichment and
Christian Gu et al.
Chemistry & biology, 20(4), 541-548 (2013-04-23)
Chemical probes have great potential for identifying functional residues in proteins in crude proteomes. Here we studied labeling sites of chemical probes based on sulfonyl fluorides (SFs) on plant and animal proteomes. Besides serine proteases and many other proteins, SF-based
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej