おすすめの製品
product name
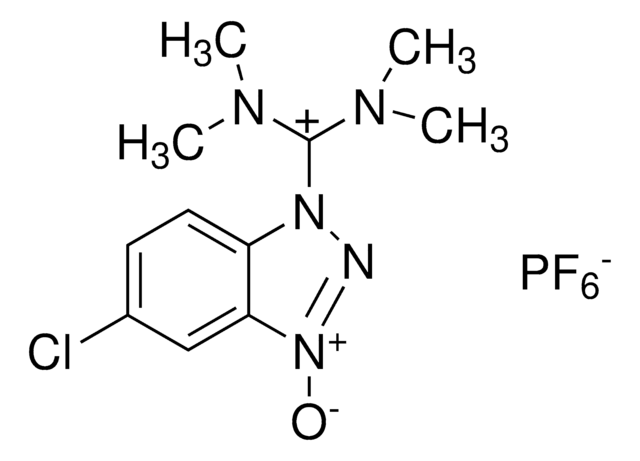
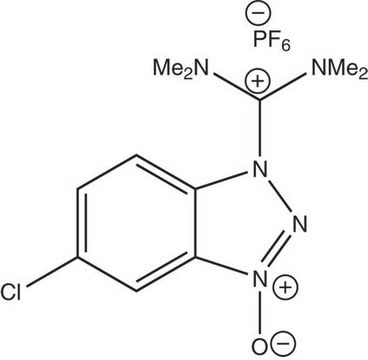
HBTU, 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate Novabiochem®
品質水準
製品種目
Novabiochem®
アッセイ
≥99.0% (HPLC)
形状
powder
有効性
>2000 mg/kg LD50, oral (Rat)
反応適合性
reaction type: Coupling Reactions
メーカー/製品名
Novabiochem®
pH
4.1 (1.6 g/L in H2O)
mp
250 °C
溶解性
1.6 g/L
アプリケーション
peptide synthesis
保管温度
2-8°C
InChI
1S/C11H16N5O/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16/h5-8H,1-4H3/q+1
InChI Key
CLZISMQKJZCZDN-UHFFFAOYSA-N
詳細
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents
Literature references
[1] R. Knorr, et al. (1989) Tetrahedron Lett., 30, 1927.
[2] M. S. Bernatowicz, et al. (1989) Tetrahedron Lett., 30, 4645.
[3] D. Ambrosius, et al. (1989) Biol. Chem. Hoppe-Seyler, 370, 217.
[4] C. G. Fields, et al. (1991) Pept. Res., 4, 95.
[5] A. G. Beck-Sickinger, et al. (1991) Pept. Res., 4, 88.
[6] G. E. Reid, et al. (1992) Anal. Biochem., 200, 301.
[7] G. B. Fields, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 1st International Symposium′, R. Epton (Eds), SPCC UK Ltd., Birmingham, 1990, pp. 241.
[8] P. A. Baybayan, et al. in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 566.
[9] J. J. Dudash, et al. (1993) Synth. Commun., 23, 349.
アプリケーション
- Synthesis of Quinoxaline Derivatives Using HBTU: A study highlighting the use of HBTU as a Lewis acid catalyst for synthesizing quinoxaline derivatives, presenting a mild and green protocol (Popatkar and Meshram, 2020).
- Efficient Conversion of Carboxylic Acids into Benzimidazoles: Details an HBTU-promoted methodology for converting carboxylic acids into benzimidazoles in a one-pot strategy (Barasa and Yoganathan, 2018).
- Synthesis of Malonyl-linked Glycoconjugates: Discusses the use of HBTU in the synthesis of glycoconjugates, comparing its efficiency with other reagents (Nörrlinger et al., 2016).
関連事項
アナリシスノート
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
法的情報
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Skin Sens. 1A
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
資料
Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)

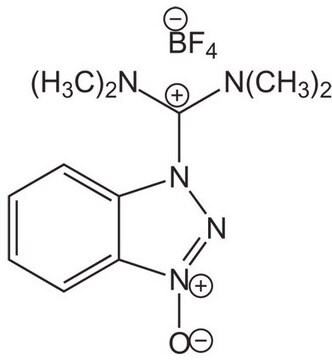

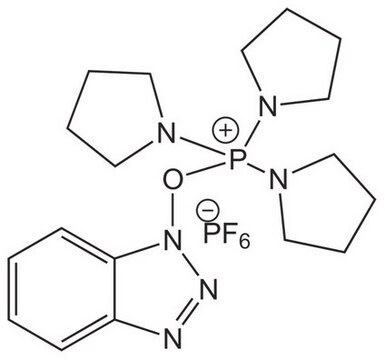
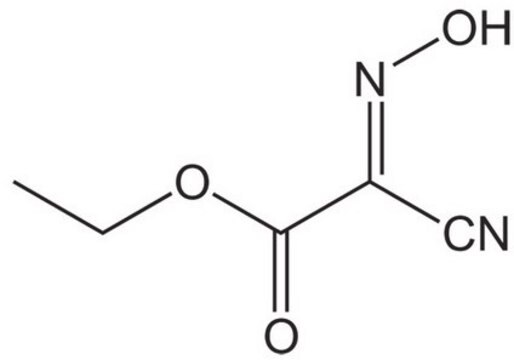
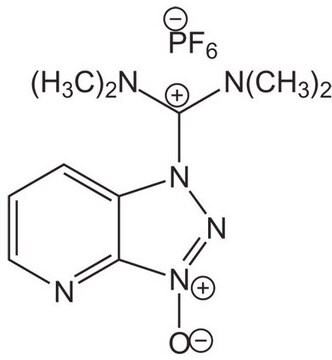

![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)