おすすめの製品
フォーム
powder
品質水準
mp
156-161 °C
官能基
phosphine
sulfonamide
保管温度
2-8°C
SMILES記法
O=S(N1C[C@H]2[P@@](C3=CC=C(OC)C=C3)C[C@@H]1C2)(C4=CC=C(C)C=C4)=O
InChI
1S/C19H22NO3PS/c1-14-3-9-19(10-4-14)25(21,22)20-12-18-11-15(20)13-24(18)17-7-5-16(23-2)6-8-17/h3-10,15,18H,11-13H2,1-2H3
InChI Key
RMFZMNUGIFSNIE-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
The bicyclic, chiral phosphine was developed by the Kwon Research Group.Its initial applications were for asymmetric [3+2] annulation between allenes and imines as well as the first examples of phosphine-catalyzed asymmetric syntheses of 1,2,3,5-substituted pyrrolines. Along with nucleophilic organocatalysis, the P-chiral phosphines may also find utility in asymmetric transition-metal catalysis.
その他情報
Hydroxyproline-Derived Pseudoenantiomeric [2.2.1] Bicyclic Phosphines: Asymmetric Synthesis of (+)- and (-)-Pyrrolines
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Lingchao Cai et al.
Journal of the American Chemical Society, 138(10), 3298-3301 (2016-02-26)
Described herein is a catalytic asymmetric total synthesis of (-)-actinophyllic acid, with the key step being a chiral phosphine-catalyzed [3 + 2] annulation between an imine and an allenoate to form a pyrroline intermediate in 99% yield and 94% ee.
Yiting Gu et al.
Journal of the American Chemical Society, 137(19), 6400-6406 (2015-04-24)
Two classes of phosphine-catalyzed addition/cycloaddition domino reactions of β'-acetoxy allenoate 1 have been developed. The reaction of 1 with 2-acyl-3-methyl-acrylonitrile 2 readily occurs to give 2-oxabicyclo[3.3.1]nonane 3, furnishing the β'-addition/[4 + 4] cycloaddition domino sequence. In this sequence, β'C of
Ian P Andrews et al.
Chemical science, 3(8), 2510-2514 (2012-07-17)
In this study we performed the total synthesis of the terpene indole alkaloid (+)-ibophyllidine through a pathway involving asymmetric phosphine catalysis, with our novel l-4-hydroxyproline-derived chiral phosphine mediating the key [3 + 2] annulation. Hydrogenation of the [3 + 2]
Christopher E Henry et al.
Journal of the American Chemical Society, 136(34), 11890-11893 (2014-08-08)
We have prepared two new diastereoisomeric 2-aza-5-phosphabicyclo[2.2.1]heptanes from naturally occurring trans-4-hydroxy-L-proline in six chemical operations. These syntheses are concise and highly efficient, with straightforward purification. When we used these chiral phosphines as catalysts for reactions of γ-substituted allenoates with imines
資料
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)![Exo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![Exo-4-anisole Kwon [2.2.1] bicyclic phosphine](/deepweb/assets/sigmaaldrich/product/structures/114/753/2a544671-b0e0-4556-8dc3-46c126d6c8ab/640/2a544671-b0e0-4556-8dc3-46c126d6c8ab.png)
![Endo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/060/39f2b621-f484-49c3-b692-cdb610e8c517/640/39f2b621-f484-49c3-b692-cdb610e8c517.png)
![Exo-2-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/324/907/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4/640/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4.png)
![Endo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/170/551/a39b471a-5427-43d5-9420-111b638ec1ac/640/a39b471a-5427-43d5-9420-111b638ec1ac.png)
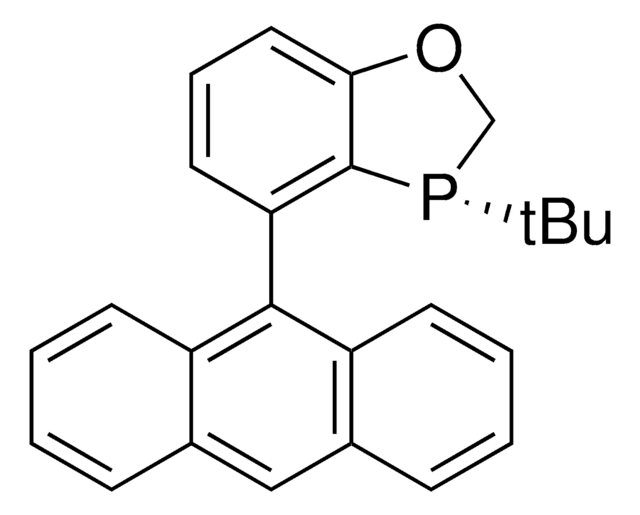
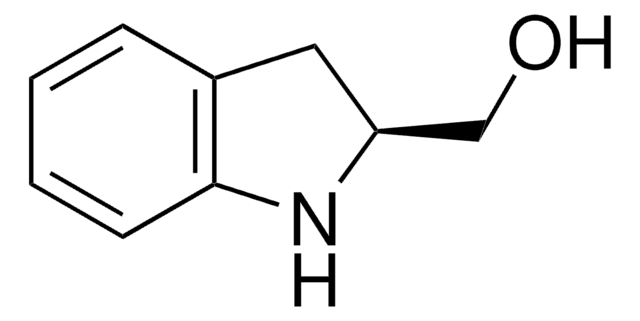
![(R)-(–)-4,12-ビス(ジフェニルホスフィノ)-[2.2]-パラシクロファン 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)
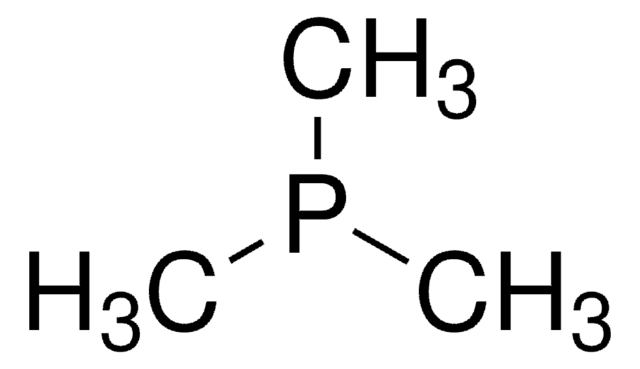
![Exo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/376/476/5be457d0-df63-4a3d-823c-9a8002a7a813/640/5be457d0-df63-4a3d-823c-9a8002a7a813.png)
