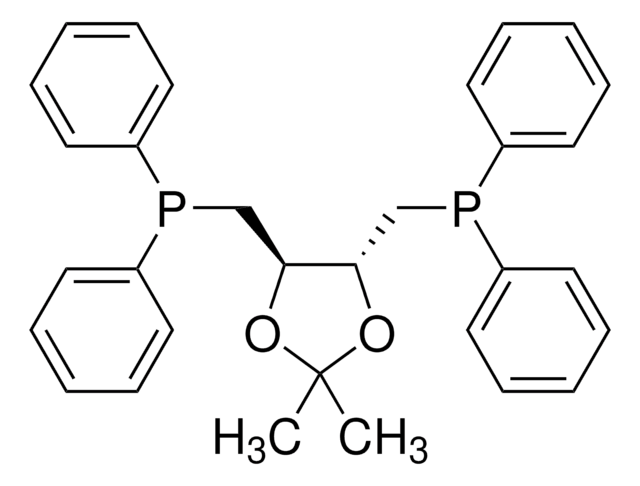
おすすめの製品
品質水準
アッセイ
95% (HPLC)
形状
powder
保管条件
under inert gas
mp
96-101 °C
官能基
phosphine
sulfonamide
保管温度
2-8°C
SMILES記法
O=S(N1C[C@@]2([H])[P@@](C3=CC=CC=C3)C[C@]1([H])C2)(C4=CC=C(C)C=C4)=O
InChI
1S/C18H20NO2PS/c1-14-7-9-18(10-8-14)23(20,21)19-12-17-11-15(19)13-22(17)16-5-3-2-4-6-16/h2-10,15,17H,11-13H2,1H3/t15-,17-,22?/m0/s1
InChI Key
BWXYDSDVFPJTFY-TWMUNHRGSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
The bicyclic, chiral phosphine was developed by the Kwon Research Group.Its initial applications were for asymmetric [3+2] annulation between allenes and imines as well as the first examples of phosphine-catalyzed asymmetric syntheses of 1,2,3,5-substituted pyrrolines. Along with nucleophilic organocatalysis, the P-chiral phosphines may also find utility in asymmetric transition-metal catalysis.
その他情報
Hydroxyproline-Derived Pseudoenantiomeric [2.2.1] Bicyclic Phosphines: Asymmetric Synthesis of (+)- and (-)-Pyrrolines
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Lingchao Cai et al.
Journal of the American Chemical Society, 138(10), 3298-3301 (2016-02-26)
Described herein is a catalytic asymmetric total synthesis of (-)-actinophyllic acid, with the key step being a chiral phosphine-catalyzed [3 + 2] annulation between an imine and an allenoate to form a pyrroline intermediate in 99% yield and 94% ee.
Yiting Gu et al.
Journal of the American Chemical Society, 137(19), 6400-6406 (2015-04-24)
Two classes of phosphine-catalyzed addition/cycloaddition domino reactions of β'-acetoxy allenoate 1 have been developed. The reaction of 1 with 2-acyl-3-methyl-acrylonitrile 2 readily occurs to give 2-oxabicyclo[3.3.1]nonane 3, furnishing the β'-addition/[4 + 4] cycloaddition domino sequence. In this sequence, β'C of
Ian P Andrews et al.
Chemical science, 3(8), 2510-2514 (2012-07-17)
In this study we performed the total synthesis of the terpene indole alkaloid (+)-ibophyllidine through a pathway involving asymmetric phosphine catalysis, with our novel l-4-hydroxyproline-derived chiral phosphine mediating the key [3 + 2] annulation. Hydrogenation of the [3 + 2]
Christopher E Henry et al.
Journal of the American Chemical Society, 136(34), 11890-11893 (2014-08-08)
We have prepared two new diastereoisomeric 2-aza-5-phosphabicyclo[2.2.1]heptanes from naturally occurring trans-4-hydroxy-L-proline in six chemical operations. These syntheses are concise and highly efficient, with straightforward purification. When we used these chiral phosphines as catalysts for reactions of γ-substituted allenoates with imines
資料
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)![Exo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![Exo-4-anisole Kwon [2.2.1] bicyclic phosphine](/deepweb/assets/sigmaaldrich/product/structures/114/753/2a544671-b0e0-4556-8dc3-46c126d6c8ab/640/2a544671-b0e0-4556-8dc3-46c126d6c8ab.png)
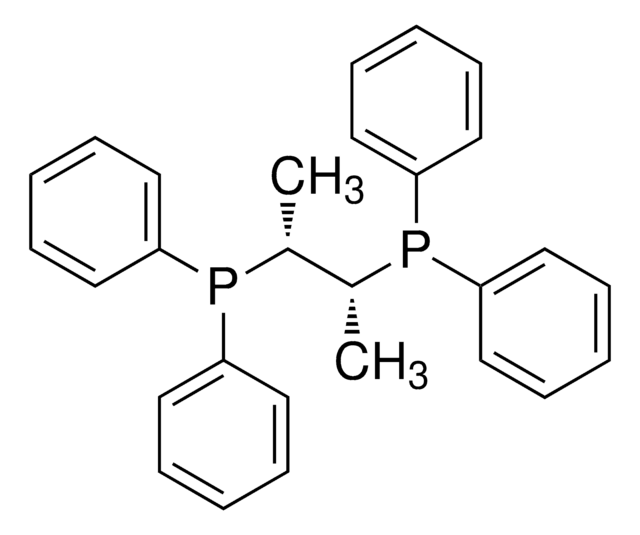
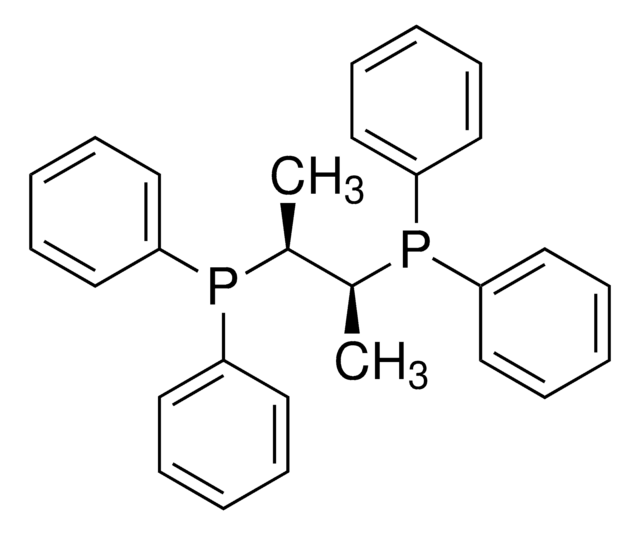

![Endo-4-Methoxyphenyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/404/012/38bdf2c6-e120-483d-8c3c-8fa3b328963c/640/38bdf2c6-e120-483d-8c3c-8fa3b328963c.png)