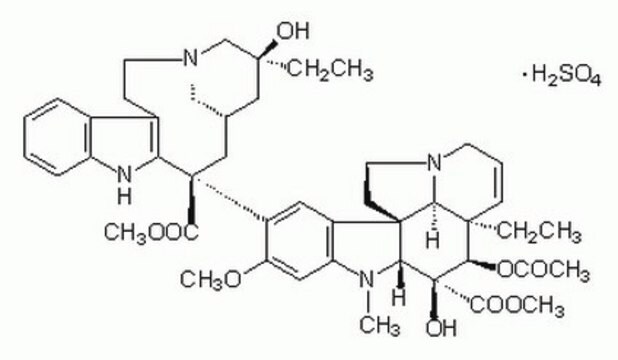
V1377
Vinblastine sulfate salt
≥97% (HPLC), powder, plant alkaloid
Sinônimo(s):
VLB, Vincaleukoblastine sulfate salt
About This Item
Produtos recomendados
product name
Vinblastine sulfate salt, ≥97% (HPLC)
Nível de qualidade
Ensaio
≥97% (HPLC)
forma
(powder or amorphous or crystalline powder)
cor
white to light yellow
pf
267 °C (dec.) (lit.)
absorção
14 at 270 nm in 0.1 M phosphate buffer at 1 mM
16.2 at 259 nm in ethanol at 1 mM
53.7 at 214 nm in ethanol at 1 mM
espectro de atividade do antibiótico
neoplastics
Modo de ação
DNA synthesis | interferes
originador
Eli Lilly
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
OS(O)(=O)=O.[H][C@@]12CN(CCc3c([nH]c4ccccc34)[C@@](C1)(C(=O)OC)c5cc6c(cc5OC)N(C)[C@@]7([H])[C@](O)([C@H](OC(C)=O)[C@]8(CC)C=CCN9CC[C@]67[C@]89[H])C(=O)OC)C[C@](O)(CC)C2
InChI
1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37-,38+,39+,42-,43+,44+,45-,46-;/m0./s1
chave InChI
KDQAABAKXDWYSZ-PNYVAJAMSA-N
Informações sobre genes
human ... TBCC(6903) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- as a microtubule depolymerizing drug for the synchronization of human cell lines in G2/M phase
- as a multidrug resistance screening substrate in human colon cancer cell line (HCT116) cell line
- as an antimicrotubule agent in sub perineural glia of Drosophila brain
Ações bioquímicas/fisiológicas
Características e benefícios
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Muta. 2 - Repr. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
We presents an article on ABC Transporters and Cancer Drug Resistance
Conteúdo relacionado
Discover Bioactive Small Molecules for ADME/Tox
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica