T7765
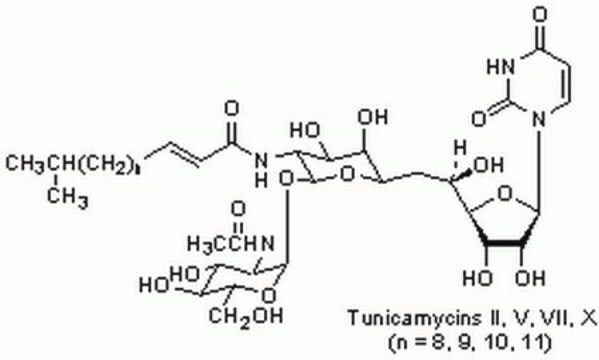
Tunicamycin
from Streptomyces sp., ≥98% (HPLC), powder, N-acetylglucosamine transferase inhibitor
About This Item
Produtos recomendados
Nome do produto
Tunicamycin from Streptomyces sp.,
Nível de qualidade
solubilidade
95% ethanol: soluble 1 mg/mL, clear to faintly hazy
THF: soluble <1 mg/mL
dioxane: soluble <1 mg/mL
DMF: soluble >10 mg/mL
pyridine: >10 mg/mL
DMSO: soluble 4.9-5.1 mg/mL, clear to slightly hazy, colorless to yellow
methanol: slightly soluble 4.9-5.1 mg/mL
methanol: soluble 4.9-5.1 mg/mL, clear to slightly hazy, colorless to yellow
acetone: insoluble
aqueous base: insoluble
chloroform: insoluble
ethyl acetate: insoluble
espectro de atividade do antibiótico
fungi
viruses
Modo de ação
protein synthesis | interferes
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
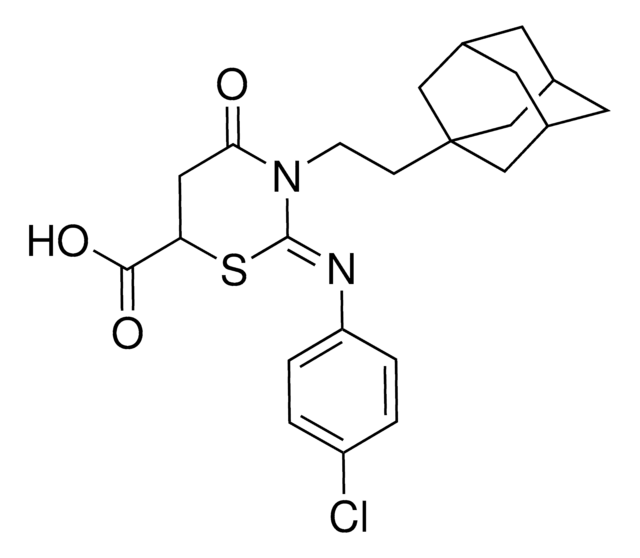
CC(C)CCCCCCCC\C=C\C(=O)N[C@@H]1[C@@H](O)[C@@H](O)[C@@H](C[C@@H](O)[C@H]2O[C@H]([C@H](O)[C@@H]2O)N3C=CC(=O)NC3=O)O[C@H]1O[C@@H]4O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]4NC(C)=O
InChI
1S/C37H60N4O16/c1-18(2)12-10-8-6-4-5-7-9-11-13-23(45)39-26-30(50)27(47)21(54-36(26)57-35-25(38-19(3)43)29(49)28(48)22(17-42)55-35)16-20(44)33-31(51)32(52)34(56-33)41-15-14-24(46)40-37(41)53/h11,13-15,18,20-22,25-36,42,44,47-52H,4-10,12,16-17H2,1-3H3,(H,38,43)(H,39,45)(H,40,46,53)/b13-11+/t20-,21-,22+,25+,26-,27+,28+,29+,30-,31+,32-,33-,34-,35+,36+/m1/s1
chave InChI
YJQCOFNZVFGCAF-WPTOCQRYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Nota de preparo
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 1 Oral
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Inhibition of Cell Wall Biosynthesis by Antibiotics
Conteúdo relacionado
We offer agonists, antagonists, modulators and other bioactive small molecules for immune system signaling target identification and validation, as well as a variety of antibiotics, antivirals, and antifungals.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica