SAE0097
D-2-Hydroxyglutarate Dehydrogenase (D2HGDH) from Acidaminococcus fermentans
recombinant, expressed in E. coli, aqueous solution
Sinônimo(s):
D2HGDH, HGDH, L-2-hydroxyglutarate dehydrogenase
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Número da licença da enzima:
1.1. 99.2
Código UNSPSC:
12352202
NACRES:
NA.54
Produtos recomendados
recombinante
expressed in E. coli
Ensaio
≥95% (SDS-PAGE)
Formulário
aqueous solution
atividade específica
≥1000 units/mg protein
nº de adesão UniProt
Condições de expedição
wet ice
temperatura de armazenamento
−20°C
Descrição geral
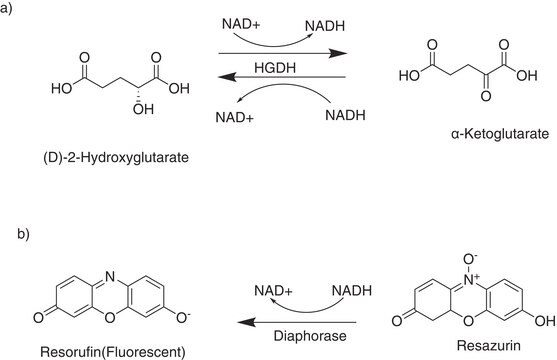
D-2-Hydroxyglutarate Dehydrogenase (D2HGDH) is a member of the D-2-hydroxyacid NAD+ dependent dehydrogenase family of proteins. D2HGDH catalyzes the conversion of α-ketoglutarate (α--KG) to D-2-hydroxyglutarate (D2HG), coupled to the oxidation of NADH to NAD+ .
The crystal structure of D2HGDH from Acidaminococcus fermentans has been reported. D2HGDH from Acidaminococcus fermentans has been used in several enzymatic assays, such as:
The crystal structure of D2HGDH from Acidaminococcus fermentans has been reported. D2HGDH from Acidaminococcus fermentans has been used in several enzymatic assays, such as:
- A continuous spectrophotometric assay to measure the activity of aminotransferases, based on the transamination of a keto compound and L-glutamate, which yields a corresponding amino compound and 2-oxoglutarate.
- Determination of D2HG levels in biological fluids such as serum, urine, cell culture supernatants, and cell or tissue lysates.
- A coupled assay system to measure branched-chain amino acid aminotransferase activity.
Definição da unidade
One unit of enzyme oxidizes 1 μmole of NADH to NAD+ coupled to the reduction of α-ketoglutarate to (D)-2-hydroxyglutarate per minute at 37°C at pH 8.0.
Nota de preparo
This recombinant D2HGDH product is supplied as an aqueous solution in 20 mM Trizma® buffer, pH 7.5, with 150 mM NaCl, and 10% glycerol.
Informações legais
Trizma is a registered trademark of Merck KGaA, Darmstadt, Germany
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Berta M Martins et al.
The FEBS journal, 272(1), 269-281 (2005-01-07)
NAD(+)-dependent (R)-2-hydroxyglutarate dehydrogenase (HGDH) catalyses the reduction of 2-oxoglutarate to (R)-2-hydroxyglutarate and belongs to the d-2-hydroxyacid NAD(+)-dependent dehydrogenase (d-2-hydroxyacid dehydrogenase) protein family. Its crystal structure was determined by phase combination to 1.98 A resolution. Structure-function relationships obtained by the comparison
Xuejing Yu et al.
Analytical biochemistry, 431(2), 127-131 (2012-09-25)
A continuous general spectrophotometric assay for measuring the activity of aminotransferases has been developed. It is based on the transamination of a keto compound (amino acceptor) and l-glutamate (amino donor), yielding the corresponding amino compound and 2-oxoglutarate. The rate of
Jörg Balss et al.
Acta neuropathologica, 124(6), 883-891 (2012-11-03)
Levels of (D)-2-hydroxyglutarate [D2HG, (R)-2-hydroxyglutarate] are increased in some metabolic diseases and in neoplasms with mutations in the isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) genes. Determination of D2HG is of relevance to diagnosis and monitoring of disease.
Xuejing Yu et al.
The FEBS journal, 281(1), 391-400 (2013-11-12)
Branched-chain amino acid aminotransferase (BCAT) plays a key role in the biosynthesis of hydrophobic amino acids (such as leucine, isoleucine and valine), and its substrate spectrum has not been fully explored or exploited owing to the inescapable restrictions of previous
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica