P7136
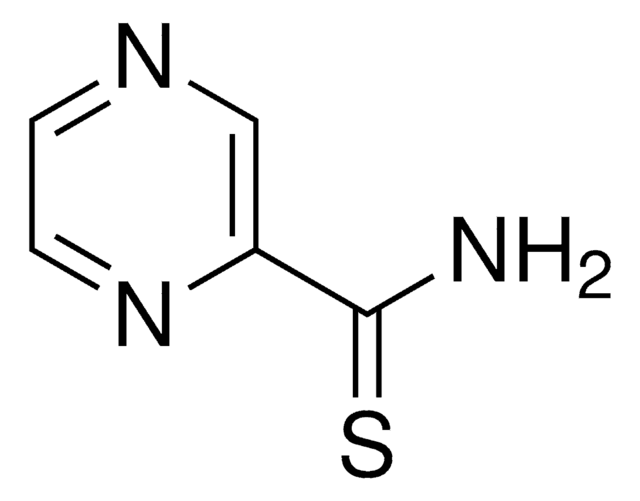
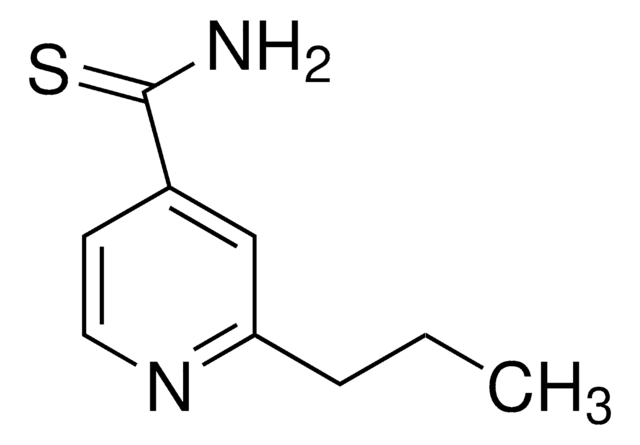
Pyrazinecarboxamide
Sinônimo(s):
Pyrazinamide, Pyrazinoic acid amide
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H5N3O
Número CAS:
Peso molecular:
123.11
Beilstein:
112306
Número CE:
Número MDL:
Código UNSPSC:
41116107
ID de substância PubChem:
NACRES:
NA.85
Produtos recomendados
Formulário
powder
Nível de qualidade
pf
189-191 °C (lit.)
espectro de atividade do antibiótico
mycobacteria
Modo de ação
cell membrane | interferes
cadeia de caracteres SMILES
NC(=O)c1cnccn1
InChI
1S/C5H5N3O/c6-5(9)4-3-7-1-2-8-4/h1-3H,(H2,6,9)
chave InChI
IPEHBUMCGVEMRF-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Pyrazinamide is used therapeutically as an antitubercular agent. Pyrazinamide is used to form polymeric copper complexes, create pyrazine carboxamide scaffolds useful as FXs inhibitors, and as a component of mycobacteria identification kits. It is used to study liver toxicity prevention and mechanisms of resistance .
Ações bioquímicas/fisiológicas
The active moiety of pyrazinamide is pyrazinoic acid (POA). POA is thought to disrupt membrane energetics and inhibit membrane transport function at acid pH in Mycobacterium tuberculosis. Iron enhances the antituberculous activity of pyrazinamide . Pyrazinamide and its analogs have been shown to inhibit the activity of purified FAS I.
Outras notas
Keep container tightly closed in a dry and well-ventilated place.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Emmanuel Chigutsa et al.
Antimicrobial agents and chemotherapy, 57(2), 789-795 (2012-11-28)
Days to positivity in automated liquid mycobacterial culture have been shown to correlate with mycobacterial load and have been proposed as a useful biomarker for treatment responses in tuberculosis. However, there is currently no quantitative method or model to analyze
Pontus Juréen et al.
Antimicrobial agents and chemotherapy, 52(5), 1852-1854 (2008-03-05)
Thirty-four pyrazinamide-resistant and 37 pyrazinamide-susceptible Mycobacterium tuberculosis complex strains were analyzed for pncA gene mutations. None of the sensitive strains had any mutations, apart from silent mutations, whereas all but one resistant strain showed pncA mutations. By using sequencing as
Akos Somoskovi et al.
The Journal of antimicrobial chemotherapy, 53(2), 192-196 (2004-01-20)
Pyrazinamide is a paradoxical frontline tuberculosis drug characterized by high in vivo sterilizing activity but poor in vitro activity. This separation in pyrazinamide activity reflects differences between the in vivo tissue environment and in vitro culture conditions. The well-known acid
S A Tasduq et al.
Human & experimental toxicology, 25(3), 111-118 (2006-04-26)
Terminalia chebula Gertn. (Combetraceae) is an important herbal drug in Ayurvedic pharmacopea. In the present study, a 95% ethanolic extract of T. chebula (fruit) (TC extract), which was chemically characterized on the basis of chebuloside II as a marker, was
Martin J Boeree et al.
American journal of respiratory and critical care medicine, 191(9), 1058-1065 (2015-02-06)
Rifampin at a dose of 10 mg/kg was introduced in 1971 based on pharmacokinetic, toxicity, and cost considerations. Available data in mice and humans showed that an increase in dose may shorten the duration of tuberculosis treatment. To evaluate the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica