B8959
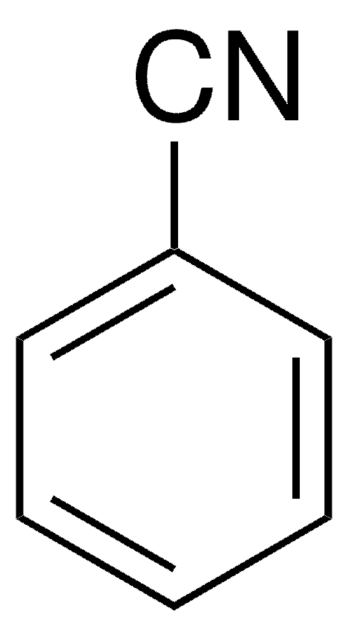
Benzonitrile
ReagentPlus®, 99%
Sinônimo(s):
Phenyl cyanide
About This Item
Produtos recomendados
grau
reagent
Nível de qualidade
linha de produto
ReagentPlus®
Ensaio
99%
Formulário
liquid
Lim. expl.
0.34-6.3 %
dilution
(for general lab use)
índice de refração
n20/D 1.528 (lit.)
p.e.
191 °C (lit.)
pf
−13 °C (lit.)
cadeia de caracteres SMILES
N#Cc1ccccc1
InChI
1S/C7H5N/c8-6-7-4-2-1-3-5-7/h1-5H
chave InChI
JFDZBHWFFUWGJE-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- An electrochemical solvent to investigate the electrochemistry, spectroscopic properties, and reactivity of a series of cobalt porphyrins with various substituents.
- Building block or starting material in various organic synthesis reactions.
- Employed in coupling reactions, such as Suzuki couplings or Heck reactions, to facilitate the formation of carbon-carbon bonds.
Informações legais
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
158.0 °F - closed cup
Ponto de fulgor (°C)
70 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica