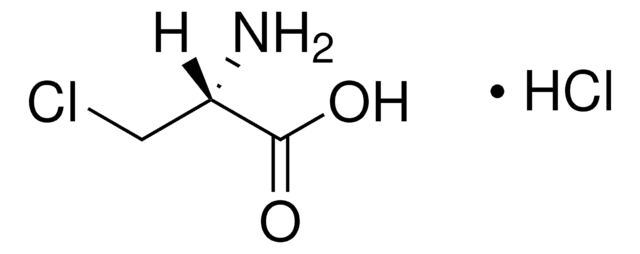
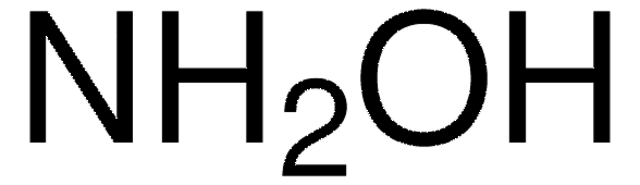C13408
O-(Carboxymethyl)hydroxylamine hemihydrochloride
98%
Sinônimo(s):
Carboxymethoxylamine hemihydrochloride, (Aminooxy)acetic acid hemihydrochloride, (Carboxymethoxy)amine hemihydrochloride, Hydroxylamine-O-acetic acid hemihydrochloride
About This Item
Produtos recomendados
fonte biológica
synthetic (organic)
Ensaio
98%
Formulário
crystalline powder
powder or crystals
pf
156 °C (dec.) (lit.)
solubilidade
water: 100 mg/mL, clear to slightly hazy, colorless to faintly yellow
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Cl.NOCC(O)=O.NOCC(O)=O
InChI
1S/2C2H5NO3.ClH/c2*3-6-1-2(4)5;/h2*1,3H2,(H,4,5);1H
chave InChI
KBXIJIPYZKPDRU-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica