A50606
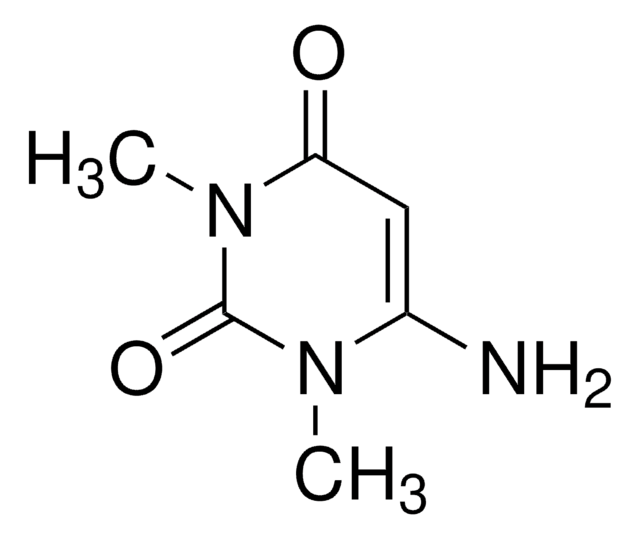
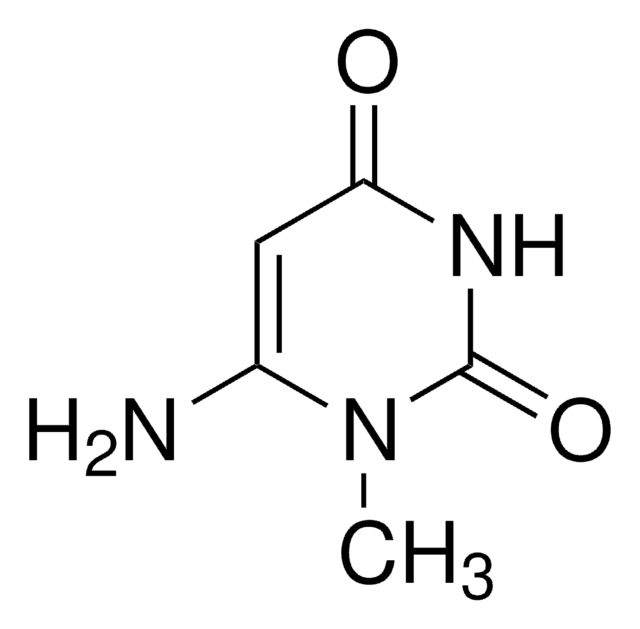
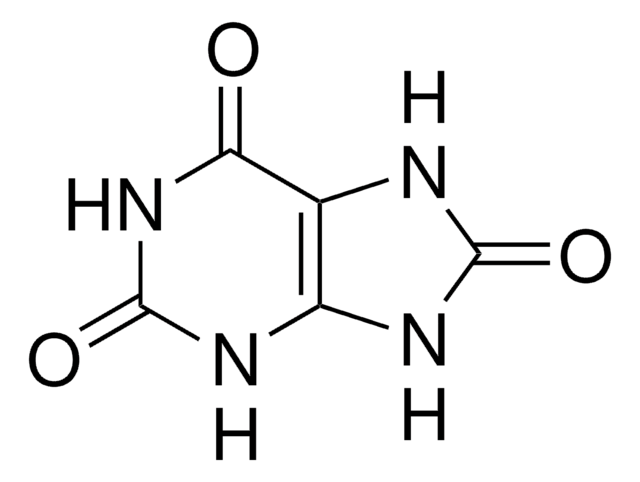
6-Aminouracil
97%
Sinônimo(s):
4-Amino-2,6-dihydroxypyrimidine, 6-Amino-2,4-pyrimidinediol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C4H5N3O2
Número CAS:
Peso molecular:
127.10
Beilstein:
120491
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
powder
pf
≥360 °C (lit.)
cadeia de caracteres SMILES
Nc1cc(O)nc(O)n1
InChI
1S/C4H5N3O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H4,5,6,7,8,9)
chave InChI
LNDZXOWGUAIUBG-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
H Sladowska et al.
Acta poloniae pharmaceutica, 53(1), 39-46 (1996-01-01)
Synthesis of 6-substituted 2,4-dioxo-1,2,3,4,5,6,7,8-octahydropyrimido[4,5-d]pyrimidines [III-VI] obtained by cyclocondensation of 1-phenyl-6-aminouracil with formaline and the primary amines is described. Compounds III, V, VI in the Mannich reaction with secondary cyclic amines yield the corresponding 3-substituted N-aminomethyl derivatives VII-X. Some of them
Kyung Mee Kim et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 877(1-2), 65-70 (2008-12-17)
Uric acid (UA) can be directly converted to allantoin enzymatically by uricase in most mammals except humans or by reaction with superoxide. UA can react directly with nitric oxide to generate 6-aminouracil and with peroxynitrite to yield triuret; both of
María-Jesús Pérez-Pérez et al.
Mini reviews in medicinal chemistry, 5(12), 1113-1123 (2005-12-27)
Thymidine Phosphorylase (TPase) catalyses the reversible phosphorolysis of pyrimidine 2'-deoxynucleosides to 2-deoxyribose-1-phosphate and their respective pyrimidine bases, including the phosphorolysis of nucleoside analogues with important antiviral or anticancer properties. Moreover, TPase, identified also as the angiogenic platelet-derived endothelial cell growth
Christine Gersch et al.
Nucleosides, nucleotides & nucleic acids, 27(8), 967-978 (2008-08-13)
The 1980 identification of nitric oxide (NO) as an endothelial cell-derived relaxing factor resulted in an unprecedented biomedical research of NO and established NO as one of the most important cardiovascular, nervous and immune system regulatory molecule. A reduction in
K Hirota et al.
Nucleic acids symposium series, (37)(37), 59-60 (1997-01-01)
Inhibitors of thymidine phosphorylase (dThdPase) are expected to suppress the growth and metastasis of tumor cells by inhibition of angiogenesis and were designed by utilizing the three dimensional structure of the enzyme. 5-Substituted 6-aminouracils (5) and 7-substituted pyrrolo[2,3-d]pyrimidine-2,4-diones (6) were
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica