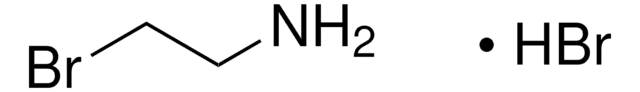
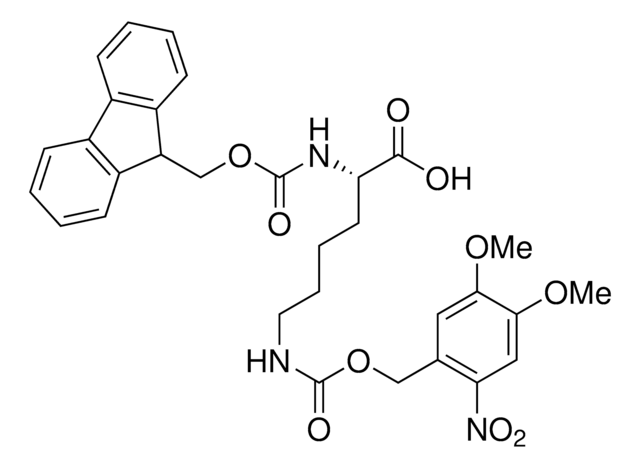
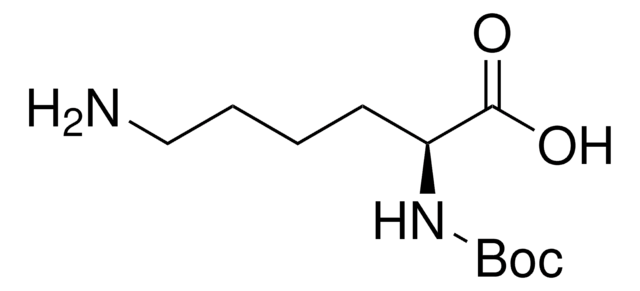
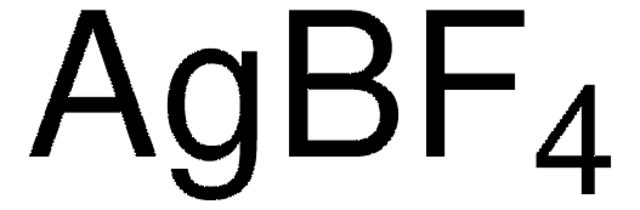913588
O-(2-Nitrobenzyl)-L-tyrosine hydrochloride
≥95%
Sinônimo(s):
(S)-2-Amino-3-(4-((2-nitrobenzyl)oxy)phenyl)propanoic acid hydrochloride, NBY, ONBY, Photo-controlled amino acid, Photocaged amino acid, Photocleavable tyrosine derivative
About This Item
Produtos recomendados
Ensaio
≥95%
Formulário
powder
disponibilidade
available only in USA
pf
205 °C (decomp.)
temperatura de armazenamento
2-8°C
InChI
1S/C16H16N2O5.ClH/c17-14(16(19)20)9-11-5-7-13(8-6-11)23-10-12-3-1-2-4-15(12)18(21)22;/h1-8,14H,9-10,17H2,(H,19,20);1H/t14-;/m0./s1
chave InChI
DRUCEARMIBXBOJ-UQKRIMTDSA-N
Aplicação
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Outras notas
Time-resolved protein activation by proximal decaging in living systems
Crystal structure of a domain-swapped photoactivatable sfGFP variant provides evidence for GFP folding pathway
Light-control of the ultra-fast Gp41-1 split intein with preserved stability of a genetically encoded photo-caged amino acid in bacterial cells
Rapid and Inexpensive Evaluation of Nonstandard Amino Acid Incorporation in Escherichia coli
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Self-react. C
Código de classe de armazenamento
5.2 - Organic peroxides and self-reacting hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![N-[2-(Fmoc-amino)-ethyl]-Gly-O-tBu hydrochloride ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/641/926/3fedc773-b21f-4419-afd5-87e20df0156a/640/3fedc773-b21f-4419-afd5-87e20df0156a.png)

![[2,2′-Bipyridine]-6-carboxylic acid hydrochloride](/deepweb/assets/sigmaaldrich/product/structures/130/786/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d/640/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d.png)