90660
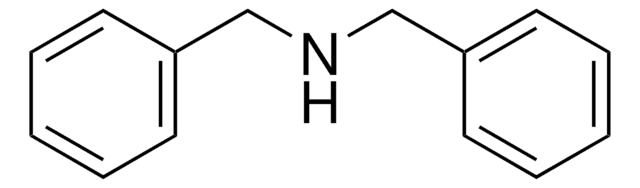
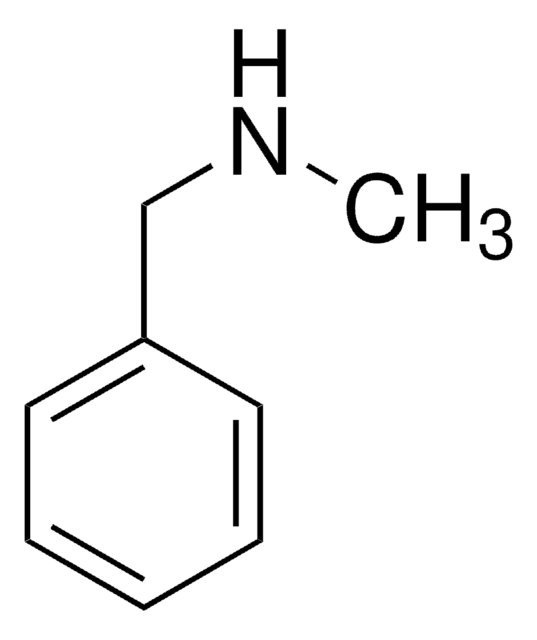
Tribenzylamine
≥99.0% (NT)
Sinônimo(s):
TBA
Faça loginpara ver os preços organizacionais e de contrato
About This Item
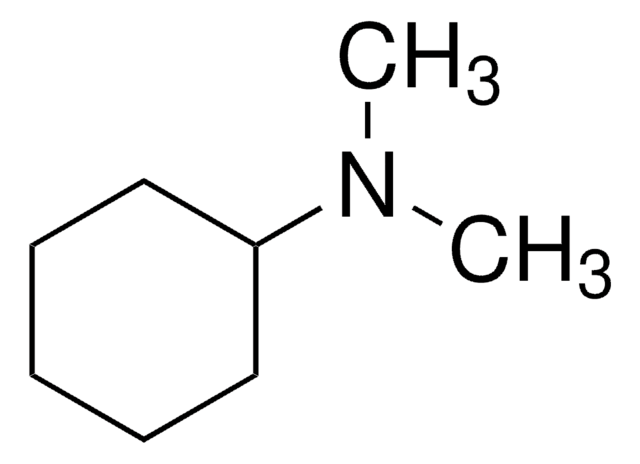
Fórmula linear:
(C6H5CH2)3N
Número CAS:
Peso molecular:
287.40
Beilstein:
2214682
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
≥99.0% (NT)
Formulário
powder
pf
91-94 °C (lit.)
grupo funcional
amine
phenyl
cadeia de caracteres SMILES
C(N(Cc1ccccc1)Cc2ccccc2)c3ccccc3
InChI
1S/C21H21N/c1-4-10-19(11-5-1)16-22(17-20-12-6-2-7-13-20)18-21-14-8-3-9-15-21/h1-15H,16-18H2
chave InChI
MXHTZQSKTCCMFG-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
Tribenzylamine (TBA) is a tertiary amine which can be used :
TBA can also undergo debenzylation in the presence of ceric ammonium nitrate (CAN) to form dibenzylamine.
- As a nitrogen group source for the reactions involving C−N bond formation.
- For the synthesis of imine i.e. N−benzylidene benzylamine by aerobic oxidative condensation.
- As an extractant for the separation and determination of Cr(VI) and Cr(III) from wastewater.
TBA can also undergo debenzylation in the presence of ceric ammonium nitrate (CAN) to form dibenzylamine.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Skin Sens. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
399.2 °F - closed cup
Ponto de fulgor (°C)
204 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Copper-Catalyzed Oxidative Amination of Benzoxazoles via C- H and C- N Bond Activation: A New Strategy for Using Tertiary Amines as Nitrogen Group Sources.
Guo S, et al.
Organic Letters, 13(3), 522-525 (2010)
Chemoselective oxidative debenzylation of tertiary N-benzyl amines.
Bull SD, et al.
Chemical Communications (Cambridge, England), 165(5), 337-338 (2000)
Highly active and selective gold catalysts for the aerobic oxidative condensation of benzylamines to imines and one-pot, two-step synthesis of secondary benzylamines.
Grirrane A, et al.
J. Catal., 264(2), 138-144 (2009)
Céline Burnier et al.
Talanta, 192, 135-141 (2018-10-24)
Nowadays, Gas Chromatography Mass Spectrometry (GC-MS) is mainly used in forensic sciences but suffers from limitations when the analysed compounds are thermally instable as it is the case for THC-A (Tetrahydrocannabinolic Acid) which is converted into Δ9-THC (Δ9-Tetrahydrocannabinol) that subsequently
Yan Qu et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 58(9-10), 640-642 (2003-10-28)
Two compounds, (p-methoxyphenyl) diphenylmethanol (1) and tribenzylamine (2), were isolated from Humulus lupulus. Their structures were established on the basis of spectral evidence (MS, IR, NMR, HMBC, HMQC, 1H-1H COSY experiments). Compounds 1 and 2 were found as natural products
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica