792764
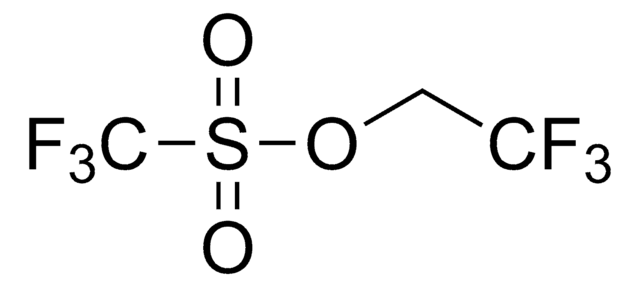
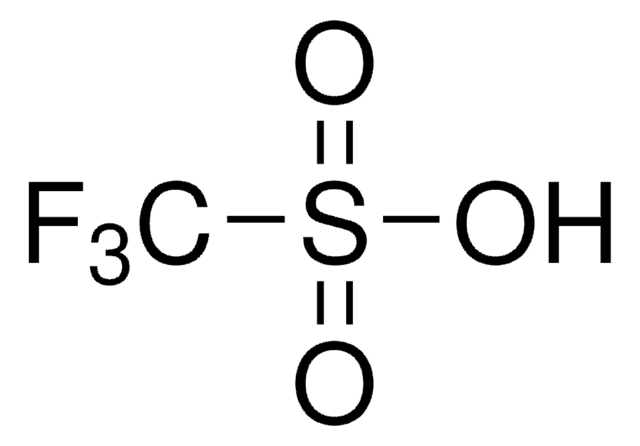
Difluoromethyl triflate
95%
Sinônimo(s):
Difluoromethyl trifluoromethanesulfonate, Trifluoromethanesulfonic acid difluoromethyl ester
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C2HF5O3S
Número CAS:
Peso molecular:
200.08
Número MDL:
Código UNSPSC:
12352108
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
liquid
densidade
1.584 g/mL at 25 °C
grupo funcional
fluoro
triflate
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
O=S(OC(F)F)(C(F)(F)F)=O
InChI
1S/C2HF5O3S/c3-1(4)10-11(8,9)2(5,6)7/h1H
chave InChI
DAANAKGWBDWGBQ-UHFFFAOYSA-N
Descrição geral
Difluoromethyl triflate (HCF2OTf) is an easy to handle, air-stable and non-ozone-depleting liquid reagent for difluoromethylation. It can be prepared by reacting trifluoromethyltrimethylsilane (TMSCF3) and triflic acid in the presence of titanium tetrachloride (TiCl4).
Aplicação
Difluoromethyl triflate (TfO-CHF2) can be used as a reagent:
It allows for a simple method toward the preparation of difluoromethyl ethers and thioethers under basic conditions from alcohols and thiols. Difluoromethyl phenols can also be obtained in a single pot from boronic acids and C-H activation of arenes.
- In difluoromethylation reaction.
- To prepare difluoromethoxylated heterocycles by reacting with hydroxylated N-based heterocycles.
- To synthesize trifluoromethylated arenes by treating with diaryliodonium salts in the presence of copper and tetrabutylammonium difluorotriphenylsilicate (TBAT).
It allows for a simple method toward the preparation of difluoromethyl ethers and thioethers under basic conditions from alcohols and thiols. Difluoromethyl phenols can also be obtained in a single pot from boronic acids and C-H activation of arenes.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
135.0 °F
Ponto de fulgor (°C)
57.22 °C
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthesis of difluoromethyl ethers with difluoromethyltriflate.
Patrick S Fier et al.
Angewandte Chemie (International ed. in English), 52(7), 2092-2095 (2013-01-12)
Difluoromethoxylation of N-Heteroaromatics
Snieckus V and Richardson P
Synfacts, 14(03), 0239-0239 (2018)
Difluoromethyl Triflate
Besset T
Encyclopedia of Reagents for Organic Synthesis, Second Edition, 203(03), 1-1 (2001)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica