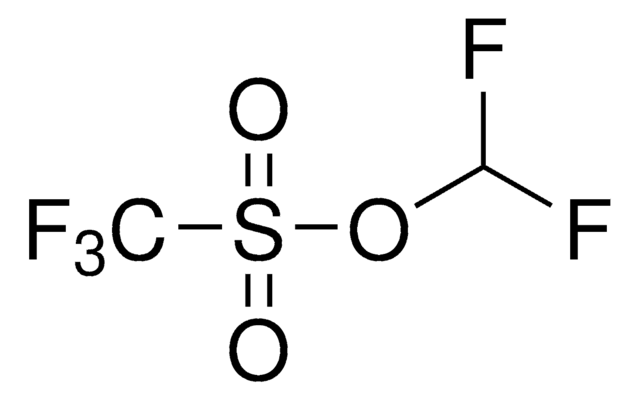
767840
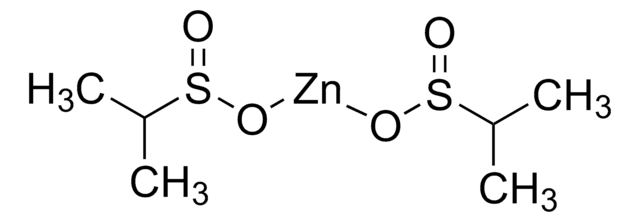
Zinc difluoromethanesulfinate
95%
Sinônimo(s):
Bis(((difluoromethyl)sulfinyl)oxy)zinc, 1,1-difluoro-methanesulfinic acid zinc salt (2:1), Baran difluoromethylation reagent, DFMS
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
solid
adequação da reação
reaction type: C-C Bond Formation
reaction type: Fluorinations
reagent type: catalyst
reaction type: C-H Activation
reagent type: diversification reagent
grupo funcional
fluoro
sulfinic acid
temperatura de armazenamento
2-8°C
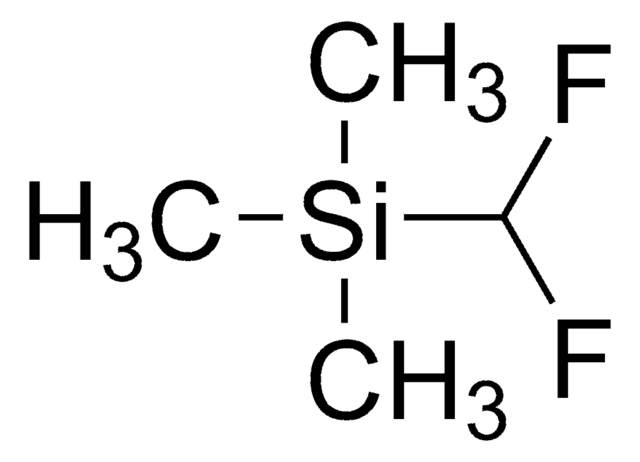
cadeia de caracteres SMILES
FC(F)S(=O)O[Zn]OS(=O)C(F)F
InChI
1S/2CH2F2O2S.Zn/c2*2-1(3)6(4)5;/h2*1H,(H,4,5);/q;;+2/p-2
chave InChI
UGEYAPVLXKEKMP-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles
DFMS is a new reagent for direct difluoromethylation of organic substrates via a radical process. This mild, operationally simple, chemoselective, and scalable difluoromethylation method is compatible with a range of nitrogen-containing heteroarene substrates of varying complexity as well as select classes of conjugated p−systems and thiols.†
A New Reagent for Direct Difluoromethylation
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
Ligação
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica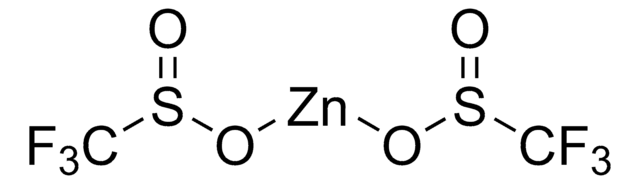

![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)