424447
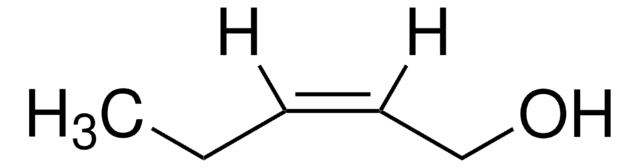
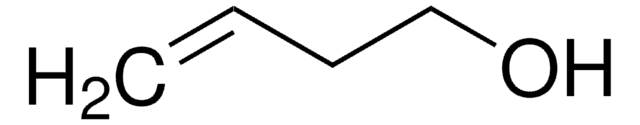
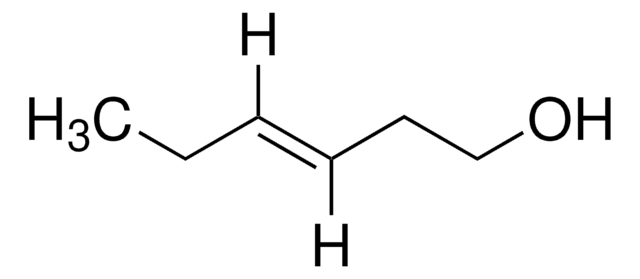
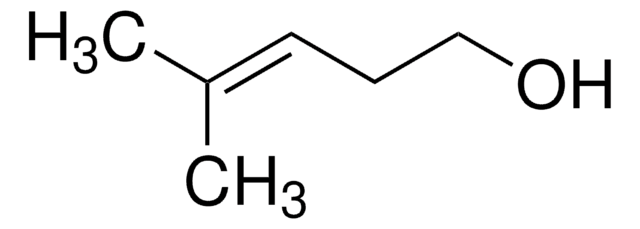
trans-2-Penten-1-ol
95%
Sinônimo(s):
(2E)-2-Penten-1-ol, (E)-Pent-2-en-1-ol, trans-2-Pentenol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C2H5CH=CHCH2OH
Número CAS:
Peso molecular:
86.13
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
95%
Formulário
liquid
índice de refração
n20/D 1.434 (lit.)
p.e.
139-139.5 °C (lit.)
densidade
0.847 g/mL at 25 °C (lit.)
grupo funcional
hydroxyl
cadeia de caracteres SMILES
CC\C=C\CO
InChI
1S/C5H10O/c1-2-3-4-5-6/h3-4,6H,2,5H2,1H3/b4-3+
chave InChI
BTSIZIIPFNVMHF-ONEGZZNKSA-N
Categorias relacionadas
Descrição geral
trans-2-Penten-1-ol is an allyl alcohol. It is one of the volatile compounds found in olive oil, cashew apple juice and fermented cucumber brines. The rate constants and product ion distributions of its reaction with H3O+, NO+ and O2.+ ions have been studied using selected ion flow tube (SIFT).
Aplicação
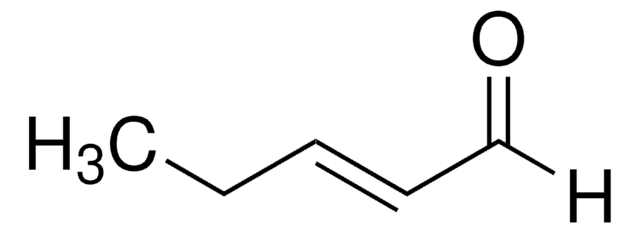
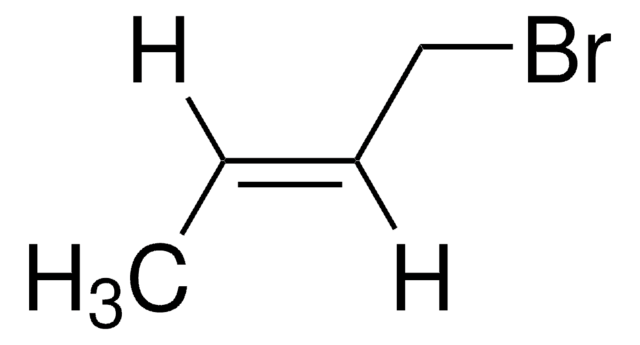
trans-2-Penten-1-ol may be used in the synthesis of the following:
- leustroducsin B
- trichloroacetimidate
- (E)-2,3,3′-trifluoro-4-(2-(trans-4-pentylcyclohexyl)ethyl)-4′-(pent-2-enyloxy)biphenyl
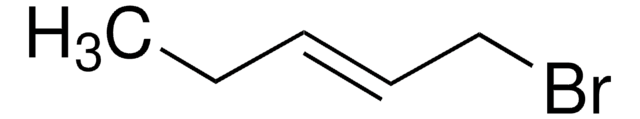
- trans-1-bromo-2-pentene
- trans-1-chloro-2-pentene
Palavra indicadora
Warning
Frases de perigo
Classificações de perigo
Flam. Liq. 3
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
118.4 °F - closed cup
Ponto de fulgor (°C)
48 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Kazuyuki Miyashita et al.
The Journal of organic chemistry, 73(14), 5360-5370 (2008-06-14)
Leustroducsin B was synthesized via a convergent route based on division of the leustroducsin molecule into three segments A, B, and C. Two coupling reactions (Julia coupling reaction and Nozaki-Hiyama-Kishi (NHK) reaction) were employed for coupling of segments A and
Suzanne D Johanningsmeier et al.
Journal of food science, 76(1), C168-C177 (2011-05-04)
A nontargeted, comprehensive 2-dimensional gas chromatography-time-of-flight mass spectrometry (GC×GC-TOFMS) method was developed for the analysis of fermented cucumber volatiles before and after anaerobic spoilage. Volatile compounds extracted by solid-phase microextraction were separated on a polyethylene glycol 1st-dimension column and 14%
Biogeneration of volatile compounds in virgin olive oil: their evolution in relation to malaxation time.
Angerosa F, et al.
Journal of Agricultural and Food Chemistry, 46(8), 2940-2944 (1998)
A selected ion flow tube study of the reactions of H3O+, NO+ and O2.+ with a series of C5, C6 and C8 unsaturated biogenic alcohols.
Schoon N, et al.
International Journal of Mass Spectrometry, 263(2-3), 127-136 (2007)
Stereoregulated synthesis of unsaturated compounds Communication 9. Stereochemistry of the reactions of aldehydes with ?, ?-unsaturated triphenylphosphonium ylides [alkylidenetriphenylphosphoranes].
Bergel'son LD, et al.
Bulletin of the Academy of Sciences of the USSR, Division of chemical science, 15(3), 468-473 (1966)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 424447-5ML | 4061837881794 |
| 424447-25ML | 4061837881787 |
| 424447-400ML |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica