390879
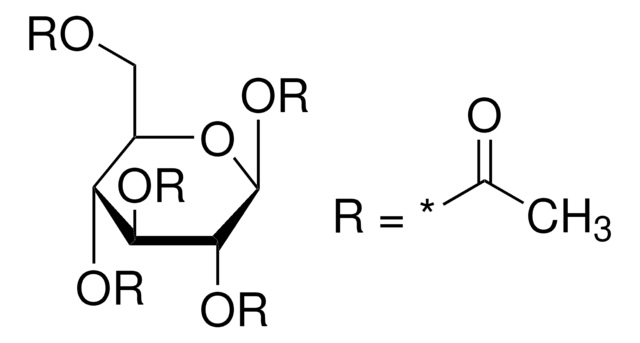
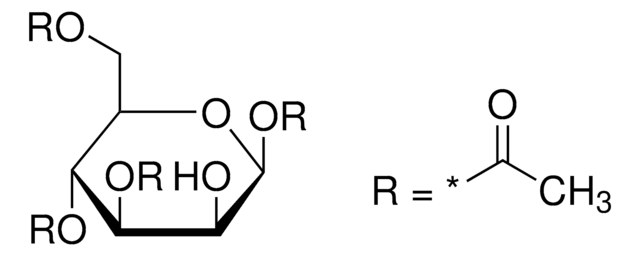
1,2,3,4-Tetra-O-acetyl-β-D-glucopyranose
98%
Sinônimo(s):
1,2,3,4-Tetra-O-acetyl-beta-D-glucose
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C14H20O10
Número CAS:
Peso molecular:
348.30
Número CE:
Número MDL:
Código UNSPSC:
12352201
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
solid
atividade óptica
[α]20/D +11°, c = 6 in chloroform
pf
126-128 °C (lit.)
cadeia de caracteres SMILES
CC(=O)O[C@@H]1O[C@H](CO)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O
InChI
1S/C14H20O10/c1-6(16)20-11-10(5-15)24-14(23-9(4)19)13(22-8(3)18)12(11)21-7(2)17/h10-15H,5H2,1-4H3/t10-,11-,12+,13-,14-/m1/s1
chave InChI
FEQXFAYSNRWXDW-RKQHYHRCSA-N
Descrição geral
1,2,3,4-Tetra-O-acetyl-β-ᴅ-glucopyranoseis a carbohydrate that is used in the synthesis of disaccharides and D-glucose6-phosphate.
Aplicação
Phosphorylated derivatives have proven valuable in the study of substrates for inositol synthase, and for the preparation of anionic surfactants.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Tetrahedron, 47, 3895-3895 (1991)
A Milius et al.
Carbohydrate research, 229(2), 323-336 (1992-05-22)
D-Glucose 3- and 6-[sodium 2-(perfluoro-hexyl or -octyl)ethyl phosphates) have been synthesized by condensation of 1,2,3,4,-tetra-O-acetyl-beta-D-glucopyranose and 1,2:5,6-di-O-isopropylidene-alpha-D-glucofuranose with 2-(perfluoroalkyl)ethylphosphoroditriazolides followed by O-deacetylation or deacetalation. The structures of the compounds were established on the basis of 1H-, 19F-, 31P-, and 13C-NMR
Beata Liberek et al.
Carbohydrate research, 341(13), 2275-2285 (2006-07-15)
The single-crystal X-ray diffraction and high-resolution 1H and 13C NMR spectral data for methyl 2,5-di-O-acetyl-beta-D-glucofuranosidurono-6,3-lactone and 1,2,5-tri-O-acetyl-beta-D-glucofuranurono-6,3-lactone are reported. The lactones were synthesized as byproducts of reactions carried out to obtain methyl 1,2,3,4-tetra-O-acetyl-D-glucopyranuronate. The conformations of these lactones in the
Yuriko Y Root et al.
Carbohydrate research, 337(21-23), 2343-2346 (2002-11-16)
The identity of the crystalline product formed by the acetylation of a mixture of methyl alpha- and beta-D-glucopyranuronates has been confirmed as being methyl 1,2,3,4-tetra-O-acetyl-beta-D-glucopyranuronate (3), which agrees with the assignment from 1H NMR. The absolute configuration of compound 3
V Flors et al.
Journal of agricultural and food chemistry, 49(5), 2569-2575 (2001-05-23)
The effects of exogenous application of a chemical mixture consisting of adipic acid monoethyl ester, furfurylamine, and 1,2,3,4-tetra-O-acetyl-beta-D-glucopyranose (FGA) on various metabolic pathways and the plant-fungus interaction have been studied in Solanaceae plants. Tomato and pepper plants were sprayed with
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica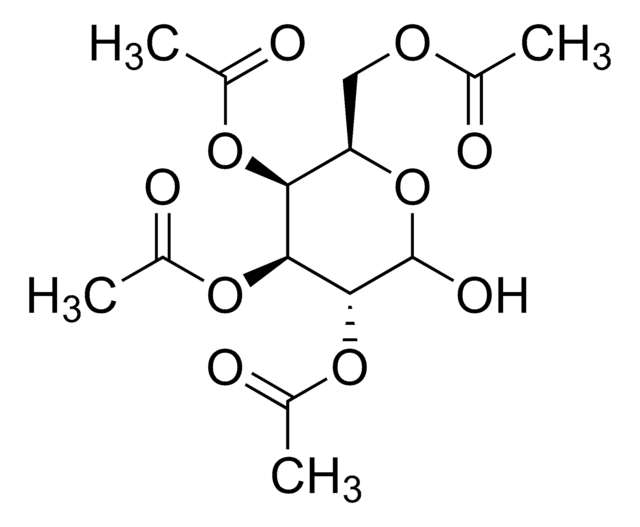

![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)