243086
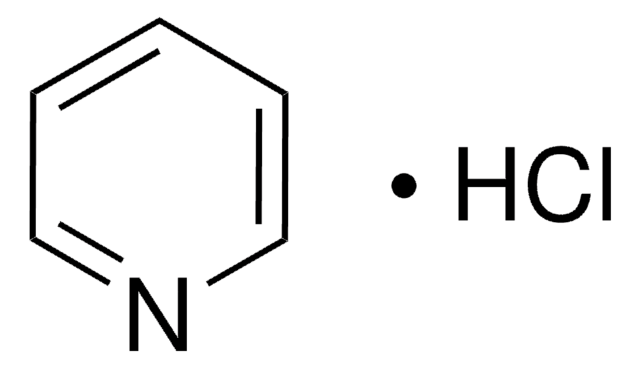
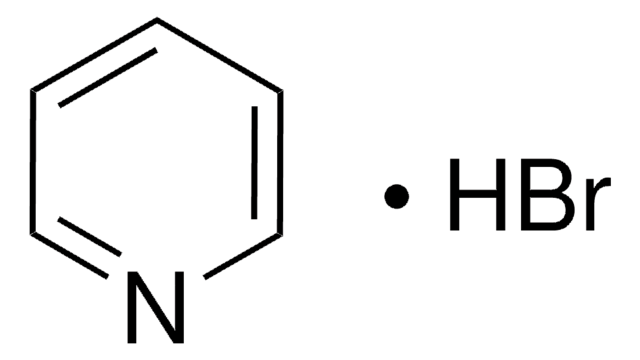
Pyridine hydrochloride
98%
Sinônimo(s):
Pyridinium chloride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H5N · HCl
Número CAS:
Peso molecular:
115.56
Beilstein:
3615340
Número CE:
Número MDL:
Código UNSPSC:
12352100
eCl@ss:
39151701
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
p.e.
222-224 °C (lit.)
pf
145-147 °C (lit.)
solubilidade
ethanol: soluble 50 mg/mL, clear, colorless to light yellow
cadeia de caracteres SMILES
Cl[H].c1ccncc1
InChI
1S/C5H5N.ClH/c1-2-4-6-5-3-1;/h1-5H;1H
chave InChI
AOJFQRQNPXYVLM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Pyridine hydrochloride is an acidic type demethylating agent used as a catalyst in deprotection of aromatic methyl ethers.
Aplicação
Pyridine hydrochloride (pyridine hydrochloride) was used in the demethylation of 4,5-dimethyl-7-methoxy-1-tetralone.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Demethylation of methyl aryl ethers using pyridine hydrochloride in solvent-free conditions under microwave irradiation.
Kulkarni PP, et al.
J. Chem. Res. Synop., 6, 394-395 (1999)
Tatyana N Sevastyanova et al.
Molecular pharmacology, 86(5), 492-504 (2014-08-13)
Metabotropic glutamate receptors (mGluRs) function as dimers. Recent work suggests that mGluR1 and mGluR5 may physically interact, but the nature and functional consequences of this relationship have not been addressed. In this study, the functional and pharmacological consequences of this
Nathalie Ségaud et al.
Inorganic chemistry, 52(2), 691-700 (2013-01-11)
We report the synthesis, characterization, and solution chemistry of a series of new Fe(II) complexes based on the tetradentate ligand N-methyl-N,N'-bis(2-pyridyl-methyl)-1,2-diaminoethane or the pentadentate ones N,N',N'-tris(2-pyridyl-methyl)-1,2-diaminoethane and N,N',N'-tris(2-pyridyl-methyl)-1,3-diaminopropane, modified by propynyl or methoxyphenyltriazolyl groups on the amino functions. Six of
Jipan Yu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(13), 4271-4277 (2013-02-13)
Efficient copper-catalyzed aerobic oxidative C-H and C-C functionalization of 1-[2-(arylamino)aryl]ethanones leading to acridones has been developed. The procedure involves cleavage of aromatic C-H and acetyl C-C bonds with intramolecular formation of a diarylketone bond. The protocol uses inexpensive Cu(O2CCF3)2 as
Ye Wei et al.
Journal of the American Chemical Society, 135(10), 3756-3759 (2013-02-27)
We describe here a [3+3]-type condensation reaction of O-acetyl ketoximes and α,β-unsaturated aldehydes that is synergistically catalyzed by a copper(I) salt and a secondary ammonium salt (or amine). This redox-neutral reaction allows modular synthesis of a variety of substituted pyridines
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 653322-25G | 4061832732794 |
| Y0000093 | 4061833794555 |
| 653322-10G | 4061832732787 |
| Y0000093-1EA | |
| 243086-100G | 4061825596013 |
| 243086-10KG | |
| 243086-25KG | |
| 243086-500G | 4061825596020 |
| 243086-5G | 4061825596037 |
| 243086-5KG |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica



![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)