179485
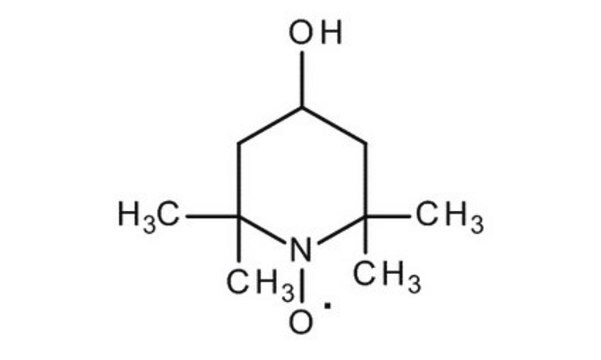
4-Oxo-TEMPO
Sinônimo(s):
4-Oxo-2,2,6,6-tetramethyl-1-piperidinyloxy, free radical
About This Item
Produtos recomendados
Formulário
solid
grupo funcional
ketone
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
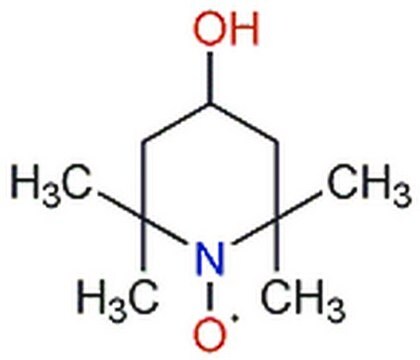
CC1(C)CC(=O)CC(C)(C)N1[O]
InChI
1S/C9H16NO2/c1-8(2)5-7(11)6-9(3,4)10(8)12/h5-6H2,1-4H3
chave InChI
WSGDRFHJFJRSFY-UHFFFAOYSA-N
Descrição geral
Aplicação
- Redox sources for anodes in lithium secondary batteries
- Free-radical biological studies
- Radical spin-trapping
- Electron paramagnetic resonance studies
- Polymer chemisty and synthesis applications
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Acute 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
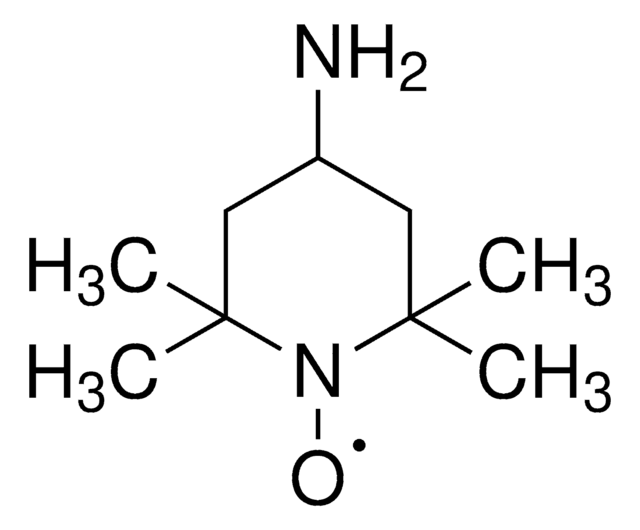
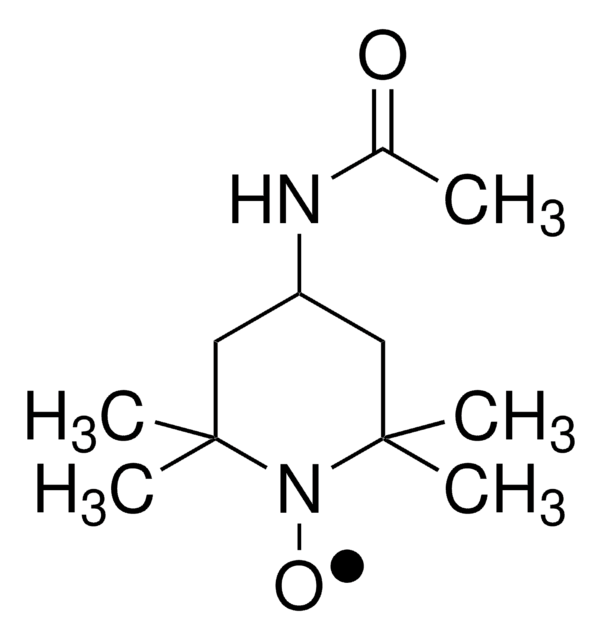
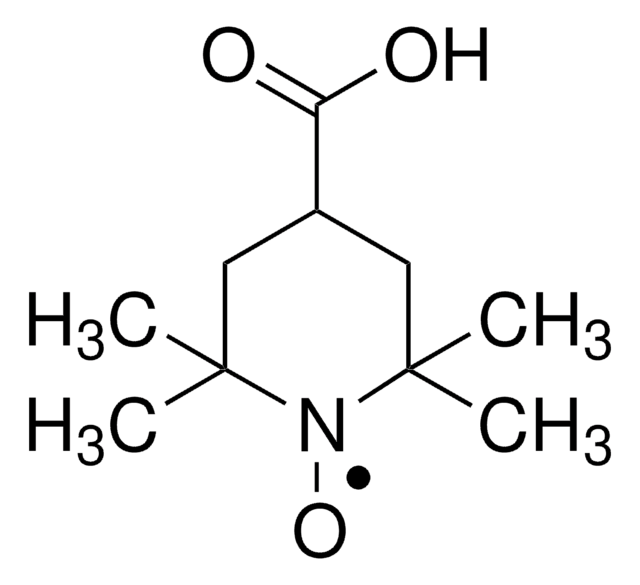
Os clientes também visualizaram
Artigos
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica

![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)