763284
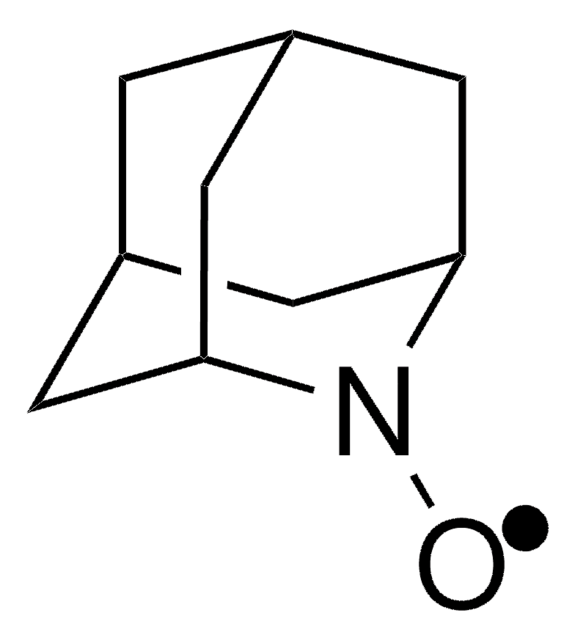
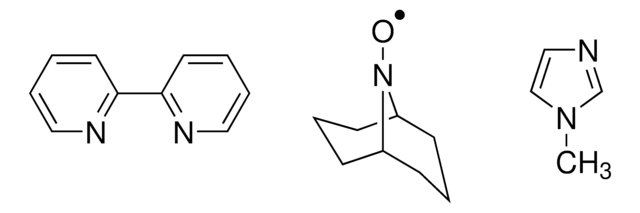
9-Azabicyclo[3.3.1]nonane N-oxyl
95%
Sinônimo(s):
9-Azabicyclo[3.3.1]nonane N-oxyl radical, ABNO
About This Item
Produtos recomendados
Ensaio
95%
Formulário
solid
adequação da reação
reagent type: ligand
características do produto alternativo mais ecológico
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
pf
65-70 °C
categoria alternativa mais ecológica
, Aligned
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
C[C@@]12CCC[C@@](C)(CCC1)N2[O]
InChI
1S/C10H18NO/c1-9-5-3-7-10(2,11(9)12)8-4-6-9/h3-8H2,1-2H3/t9-,10+
chave InChI
GGWCZKZSILYISB-AOOOYVTPSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Copper(I)/ABNO-Catalyzed Aerobic Alcohol Oxidation: Alleviating Steric and Electronic Constraints of Cu/TEMPO Catalyst Systems
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Dam. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica