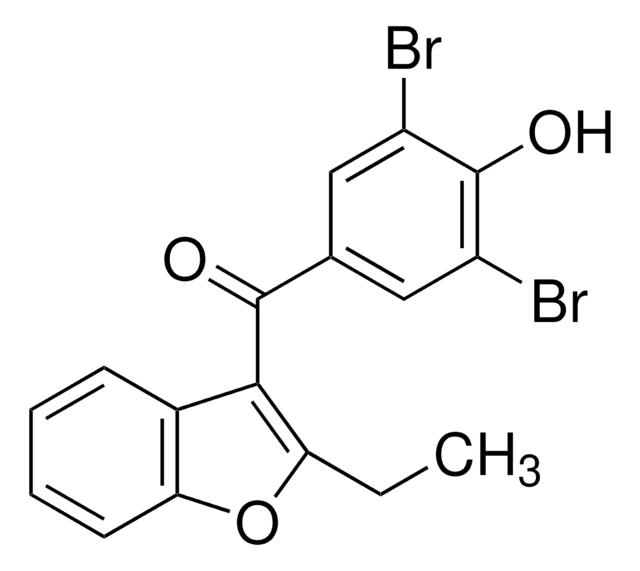
K2144
Ko143 hydrate
≥98% (HPLC), powder, BCRP inhibitor
Synonym(s):
(3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-( 2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4- b]indole-3-propanoic acid 1,1-dimethylethyl ester hydrate, Ko-143 hydrate
About This Item
Recommended Products
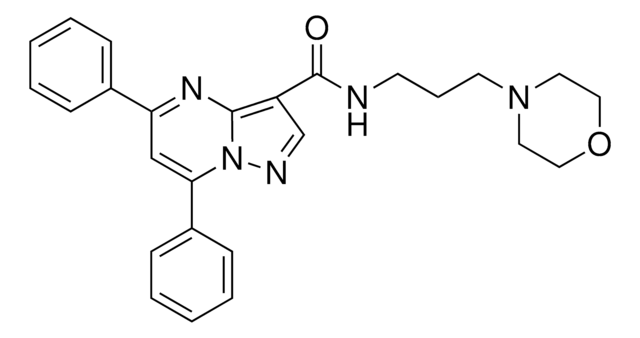
Product Name
Ko143 hydrate, ≥98% (HPLC)
assay
≥98% (HPLC)
form
powder
color
white to off-white
solubility
DMSO: >10 mg/mL
originator
GlaxoSmithKline
storage temp.
room temp
SMILES string
O.COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O
InChI
1S/C26H35N3O5.H2O/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5;/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31);1H2/t18-,20-,21-;/m0./s1
InChI key
ZXXZDXPNJJZRDC-PSLBYKFTSA-N
Application
- to determine the role of ATP-binding cassette sub-family G member 2 (ABCG2), human embryonic kidney (HEK)-C1 and HEK-ABCG2 in tumor microenvironment
- to inhibit ABCG2 for sphere formation assay
- in calcein-AM efflux inhibition to monitor multidrug resistance protein (MRP)-function in kidney
- for cell viability assay
Biochem/physiol Actions
Features and Benefits
Storage and Stability
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Protein-based drug transporters are found in most tissues including liver, kidney, intestine, and brain. These transporters are particularly important in cancer treatment and multi-drug resistance research. Understanding the specific mechanisms of tumor cell transporters is becoming an essential aspect of chemotherapeutic drug design.
Related Content
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![MK571, Sodium Salt A selective, competitive antagonist of leukotriene D4 (LTD4) (Ki = 2.1 nM for inhibition of [³H]LTD4 binding to human lung membranes).](/deepweb/assets/sigmaaldrich/product/images/110/905/114b43f4-ee35-4aba-ab41-09f068177368/640/114b43f4-ee35-4aba-ab41-09f068177368.jpg)