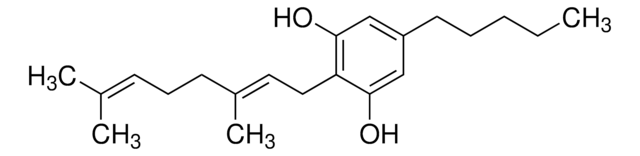
T-032
(−)-Δ8-THC solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule D (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIB (Portugal)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
cannabis testing
cannabis testing
format
single component solution
storage temp.
−20°C
InChI
1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9,12-13,16-17,22H,5-8,10-11H2,1-4H3
InChI key
HCAWPGARWVBULJ-UHFFFAOYSA-N
General description
Application
- Multi-analysis of 11 cannabinoids in biomass and extracts of different cannabis varieties by an HPLC-UV method, following the International Conference on Harmonization (ICH) Tripartite Guideline for Validation of Analytical Procedures
- Separation and estimation of 13 cannabinoids from different cannabis products using isocratic high-performance liquid chromatography (HPLC) in combination with a diode array detector (DAD)
- Gas chromatographic-mass spectrometric (GC-MS) analysis of ten main cannabinoids from hemp inflorescences following their silylation and esterification for derivatization
- Quantification of cannabidiol and Δ9-tetrahydrocannabinol in addition to the identification of minor cannabinoids from human plasma samples using ultra-performance liquid chromatography (UHPLC) combined with triple quadrupole mass spectrometry following a liquid-liquid extraction
- QuEChERS based extraction of 15 cannabinoids from 20 food product samples followed by their quantification by liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Features and Benefits
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Legal Information
Related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
51.8 °F - closed cup
flash_point_c
11.0 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
We offer a portfolio of cannabinoid CRMs such as THC and THCA, available as single component solutions or mixes, certified in accordance with ISO 17034 and ISO/IEC 17025 for accurate cannabinoid profiling and potency testing.
The cannabinoids found in the Cannabis plant, commonly referred to as marijuana, have grown in popularity for treating a variety of ailments from arthritis, glaucoma and chronic pain to malnutrition, multiple sclerosis and cancer
A comprehensive high performance liquid chromatography-diode array detection (HPLC-DAD) workflow for the analysis of 14 cannabinoids in hemp bud extracts within 10 minutes using robust Chromolith® monolithic silica HPLC columns with low column back pressure.
Protocols
Potency testing in marijuana-infused edibles is an important problem that analytical labs are facing due to the complexity of the involved matrices. Concentration of active ingredients in these edibles can range from a few parts per million to 3.5 parts per thousand. This application demonstrates the extraction and HPLC-UV analysis of the active compounds.
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
Tetrahydrocannabinolic acid A solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromenic Acid (CBCA) solution, 1.0 mg/mL in acetonitrile, certified reference material, ampule of 1 mL
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service