Kluczowe dokumenty
PHL80431
Dihydromyricetin
phyproof® Reference Substance
Synonim(y):
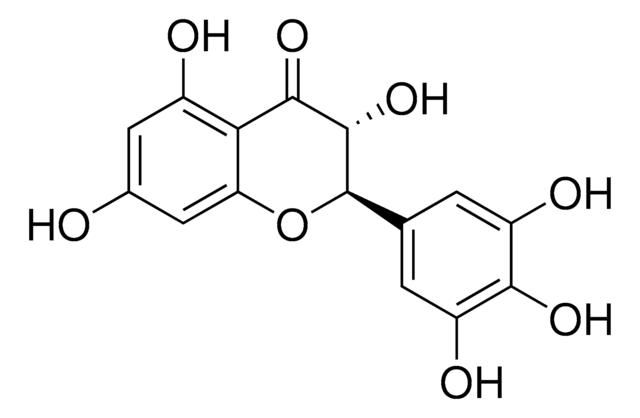
(2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one, 3,3′,4′,5,5′,7-Hexahydroxyflavanone, Ampelopsin, Ampeloptin, DHM
About This Item
Polecane produkty
klasa czystości
primary reference standard
linia produktu
phyproof® Reference Substance
Próba
≥95.0% (HPLC)
Formularz
solid
producent / nazwa handlowa
PhytoLab
ciąg SMILES
O=C1C2=C(O)C=C(O)C=C2O[C@H](C3=CC(O)=C(C(O)=C3)O)[C@H]1O
InChI
1S/C15H12O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,14-20,22H/t14-,15+/m0/s1
Klucz InChI
KJXSIXMJHKAJOD-LSDHHAIUSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Zastosowanie
- Hovenia dulcis: a Chinese medicine that plays an essential role in alcohol-associated liver disease. This review discusses the role of Hovenia dulcis, from which dihydromyricetin is derived, in treating alcohol-associated liver conditions, highlighting its mechanisms and therapeutic potentials (He YX, Liu MN, Wang YY, et al. 2024).
- Dihydromyricetin ameliorates hepatic steatosis and insulin resistance via AMPK/PGC-1α and PPARα-mediated autophagy pathway. This study explores how dihydromyricetin influences liver health, particularly in hepatic steatosis and insulin resistance, offering insights into its mechanisms through autophagy pathways (Yang Y, Qiu W, Xiao J, et al. 2024).
- Identification of dihydromyricetin as a natural DNA methylation inhibitor with rejuvenating activity in human skin. Research identifies dihydromyricetin′s potential anti-aging effects on human skin by modulating DNA methylation, which could contribute to its broader use in dermatological products (Falckenhayn C, Bienkowska A, Söhle J, et al. 2023).
- Dihydromyricetin reverses capecitabine-induced peripheral myelin dysfunction through modulation of oxidative stress. This article provides evidence of dihydromyricetin′s protective effects against peripheral myelin damage due to oxidative stress, relevant in the treatment of certain neuropathies (Fang J, Lou S, Zhou X, et al. 2024).
- The Molecular Mechanism Underlying the Therapeutic Effect of Dihydromyricetin on Type 2 Diabetes Mellitus Based on Network Pharmacology, Molecular Docking, and Transcriptomics. This comprehensive study details the molecular interactions and pathways through which dihydromyricetin could affect type 2 diabetes, providing a foundation for its application in metabolic disorder treatments (Wen X, Lv C, Zhou R, et al. 2024).
Działania biochem./fizjol.
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej