Key Documents
I1149
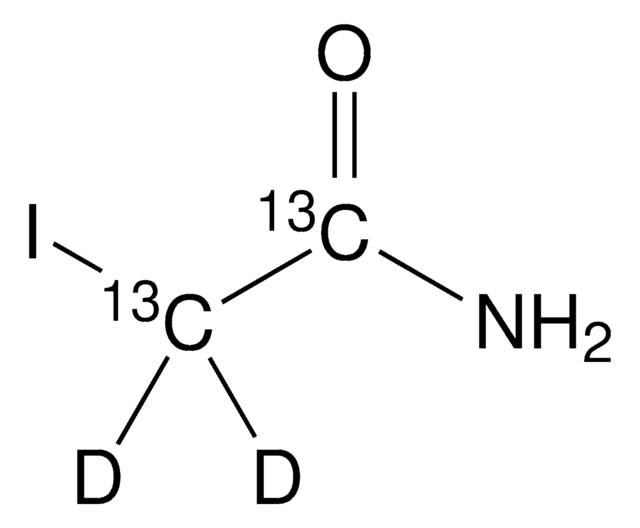
Iodoacetamide
BioUltra
Synonim(y):
2-Iodoacetamide, Monoiodoacetamide, alpha-Iodoacetamide
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic (organic)
Poziom jakości
linia produktu
BioUltra
Próba
≥99% (NMR)
Postać
powder
zanieczyszczenia
≤0.0005% Phosphorus (P)
≤0.1% Insoluble matter
mp
92-95 °C (lit.)
rozpuszczalność
H2O: 0.5 M, clear, colorless
ślady anionów
chloride (Cl-): ≤0.05%
sulfate (SO42-): ≤0.05%
ślady kationów
Al: ≤0.0005%
Ca: ≤0.0005%
Cu: ≤0.0005%
Fe: ≤0.0005%
K: ≤0.005%
Mg: ≤0.0005%
NH4+: ≤0.05%
Na: ≤0.05%
Pb: ≤0.001%
Zn: ≤0.0005%
temp. przechowywania
2-8°C
ciąg SMILES
NC(=O)CI
InChI
1S/C2H4INO/c3-1-2(4)5/h1H2,(H2,4,5)
Klucz InChI
PGLTVOMIXTUURA-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Zastosowanie
- in protein digestion for proteomic analysis
- to alkylate protein samples
- in the denaturation of cow′s milk allergens, β-lactoglobulin (BLG) and α-lactalbumin (ALA)
Działania biochem./fizjol.
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Oral - Aquatic Chronic 4 - Resp. Sens. 1 - Skin Sens. 1
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej