Kluczowe dokumenty
D9779
DL-Dithiothreitol
for molecular biology, ≥98% (HPLC), ≥99% (titration)
Synonim(y):
(±)-Dithiothreitol, rac-Dithiothreitol, Dithiothreitol, threo-1,4-Dimercapto-2,3-butanediol, Cleland’s reagent, DTT
About This Item
Polecane produkty
klasa czystości
Molecular Biology
for molecular biology
Próba
≥98% (HPLC)
≥99% (titration)
Formularz
powder
przydatność reakcji
reagent type: reductant
mp
41-44 °C (lit.)
rozpuszczalność
H2O: 50 mg/mL
ślady kationów
heavy metals (as Pb): ≤5 ppm
przydatność
suitable for molecular biology
obecność zanieczyszczeń
DNase, RNase, protease, none detected
temp. przechowywania
2-8°C
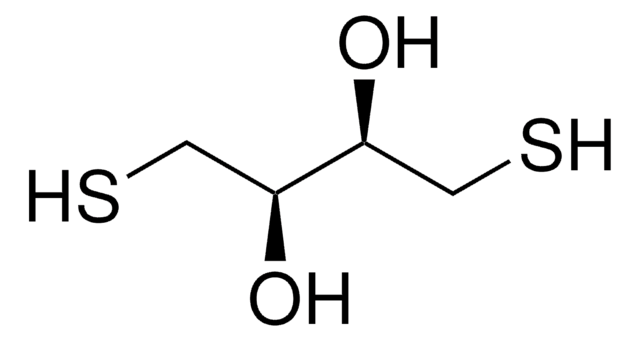
ciąg SMILES
O[C@H](CS)[C@H](O)CS
InChI
1S/C4H10O2S2/c5-3(1-7)4(6)2-8/h3-8H,1-2H2/t3-,4-/m1/s1
Klucz InChI
VHJLVAABSRFDPM-QWWZWVQMSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
It has been used:
- as a component for protein extraction in western blot analysis
- to prepare sample lysis buffer for quantitative mass spectroscopy
- as a kinase buffer component for enzyme-linked immunosorbent assay (ELISA)
Działania biochem./fizjol.
Cechy i korzyści
- Suitable for molecular biology
- RNase, DNase, Exonuclease, and Protease-free
- High purity (HPLC ≥98%)
- No heavy metal ≤5ppm
Inne uwagi
produkt podobny
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Protokoły
Follow this DDT reduction protocol to reduce disulfide bonds in thiol-modified oligonucleotides, thereby avoiding this source of oligo dimer formation.
Postępuj zgodnie z tym protokołem redukcji DDT, aby zredukować wiązania dwusiarczkowe w oligonukleotydach modyfikowanych tiolami.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej