Kluczowe dokumenty
D6513
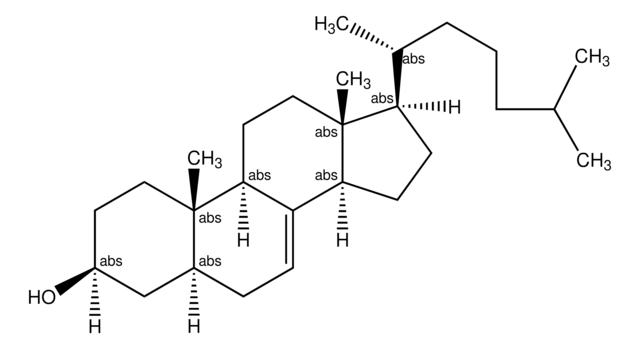
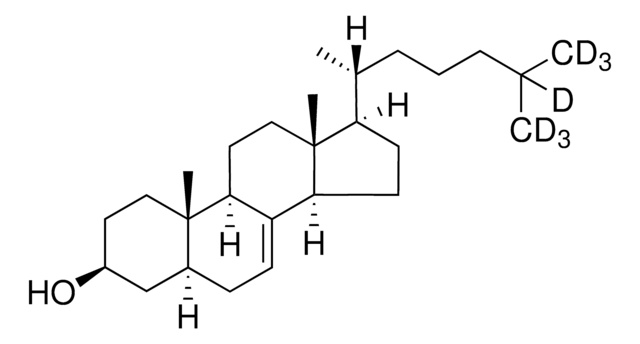
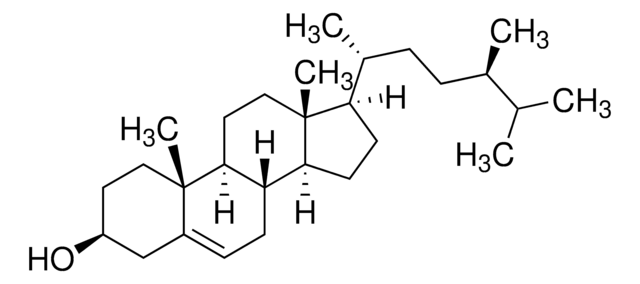
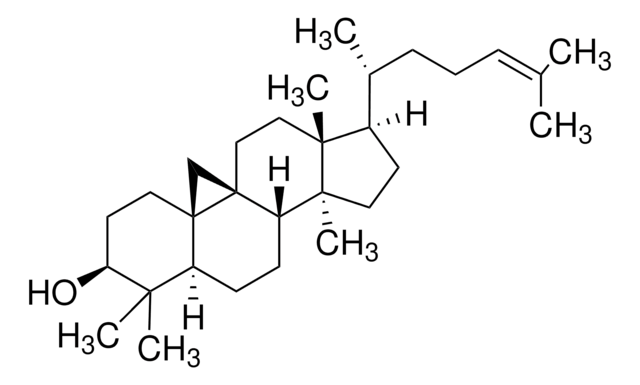
Desmosterol
≥84% (GC)
Synonim(y):
24-Dehydrocholesterol, 3β-Hydroxy-5,24-cholestadiene, 5,24-Cholestadien-3β-ol
About This Item
Polecane produkty
Próba
≥84% (GC)
Formularz
powder
temp. przechowywania
−20°C
ciąg SMILES
[H][C@@]12[C@]([C@](CC[C@H](O)C3)(C)C3=CC2)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCC=C(C)C
InChI
1S/C27H44O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h7,9,19,21-25,28H,6,8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1
Klucz InChI
AVSXSVCZWQODGV-DPAQBDIFSA-N
Powiązane kategorie
Opis ogólny
Zastosowanie
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej