Key Documents
C4290
Butyrylcholinesterase from equine serum
lyophilized powder, ≥500 units/mg protein
Synonim(y):
Acylcholine acyl-hydrolase, Choline esterase, butyryl, Pseudocholinesterase
About This Item
Polecane produkty
pochodzenie biologiczne
equine serum
Postać
lyophilized powder
aktywność właściwa
≥500 units/mg protein
skład
Protein, ≥10%
temp. przechowywania
−20°C
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
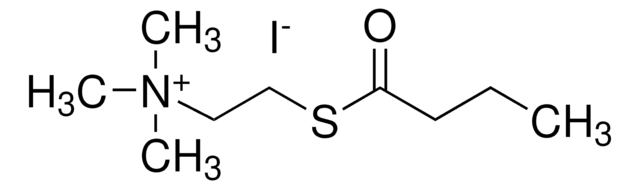
- to determine the inhibitory concentration of bupivacaine on butyrylcholinesterase
- in acetylcholinesterase (AChE)/BChE activity assay to determine the inhibitory activity of benzothiazole-piperazine compounds
Działania biochem./fizjol.
Definicja jednostki
Postać fizyczna
Komentarz do analizy
inhibitor
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Resp. Sens. 1
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej