Key Documents
E6383
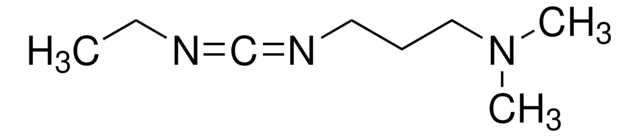
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
crystalline
Synonim(y):
EDC, N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride, EDAC, EDC hydrochloride, WSC hydrochloride
About This Item
Polecane produkty
product name
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, crystalline
Postać
crystalline
Poziom jakości
przydatność reakcji
reagent type: cross-linking reagent
reaction type: Peptide Synthesis
kolor
white to off-white
mp
110-115 °C (lit.)
rozpuszczalność
H2O: ≤100 mg/mL
Zastosowanie
advanced drug delivery
general analytical
temp. przechowywania
−20°C
ciąg SMILES
Cl.CCN=C=NCCCN(C)C
InChI
1S/C8H17N3.ClH/c1-4-9-8-10-6-5-7-11(2)3;/h4-7H2,1-3H3;1H
Klucz InChI
FPQQSJJWHUJYPU-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Beyond peptides, EDC HCl extends its influence to the construction of immunogens, where it covalently attaches haptens (small immune-response eliciting molecules) to carrier proteins, playing an instrumental role in vaccine research. The versatility of EDAC HCl further unfolds in its ability to modify nucleic acids, allowing for the labeling of DNA and RNA through their 5′ phosphate groups. This facilitates the visualization, tracking, and analysis of these essential molecules, contributing to advancements in nucleic acid research.
Additionally, EDAC HCl serves as a biomolecule bridge by acting as a crosslinker, connecting amine-reactive NHS-esters of biomolecules to carboxyl groups. This technique proves valuable in protein conjugation, enabling the creation of hybrid molecules with novel properties and functions. The underlying mechanism of EDAC HCl involves its reaction with a carboxyl group, forming an unstable intermediate that actively seeks an amine partner. The delicate balance of this reaction underscores the importance of optimizing conditions for efficient conjugation. The assistance of N-hydroxysuccinimide (NHS) further enhances EDAC HCl′s capabilities, stabilizing the intermediate and enabling two-step conjugation procedures. This additional feature provides greater flexibility and control, particularly in dealing with complex biomolecules.
Zastosowanie
- for the immobilisation of trypsin onto self-assembled monolayers (SAMs)
- as a component for the preparation of collagen matrices
- for the preparation of phosphoethanolamine(PEt)-conjugated sepharose
Działania biochem./fizjol.
Cechy i korzyści
Inne uwagi
produkt podobny
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Organy docelowe
Stomach,large intestine,lymph node
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej








![1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide](/deepweb/assets/sigmaaldrich/product/structures/414/134/4eb9c126-d7f9-4e12-9e3a-95cb077824fd/640/4eb9c126-d7f9-4e12-9e3a-95cb077824fd.png)