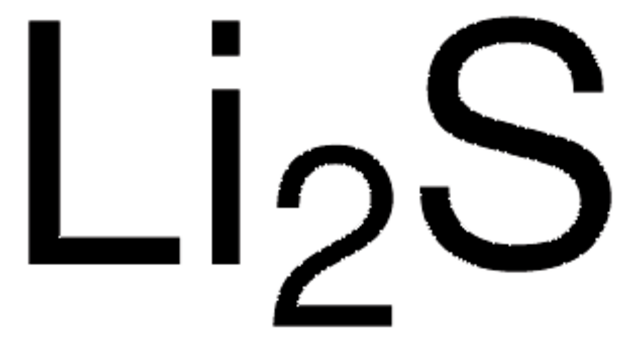Kluczowe dokumenty
930946
Lithium nitrate
battery grade, ≥99.9% trace metals basis
Synonim(y):
Sól litowa kwasu azotowego
About This Item
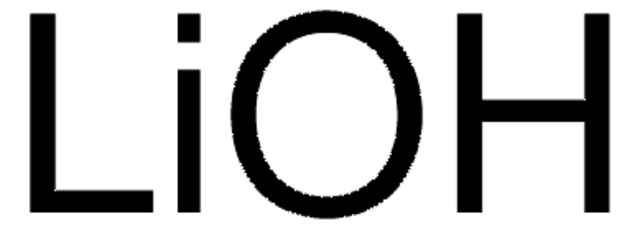
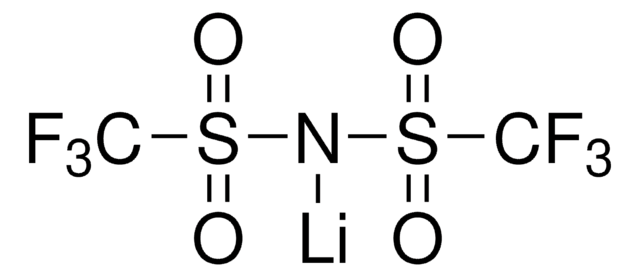
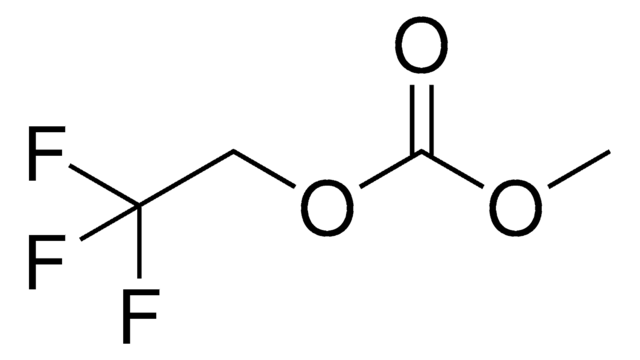
Polecane produkty
Poziom jakości
klasa czystości
battery grade
Próba
≥99.9% trace metals basis
Formularz
powder
charakterystyka ekologicznej alternatywy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
zanieczyszczenia
≤0.5 wt. % H2O
≤1000 ppm (trace metals analysis)
mp
264 °C (lit.)
rozpuszczalność
H2O: soluble (highly soluble(lit.))
acetone: soluble ((lit.))
alcohols: soluble ((lit.))
ślady anionów
chloride (Cl-): ≤500 ppm
sulfate (SO42-): ≤200 ppm
Zastosowanie
battery manufacturing
kategoria ekologicznej alternatywy
ciąg SMILES
[Li+].[O-][N+]([O-])=O
InChI
1S/Li.NO3/c;2-1(3)4/q+1;-1
Klucz InChI
IIPYXGDZVMZOAP-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Azotan litu jest wytwarzany w reakcji kwasowo-zasadowej między kwasem azotowym a węglanem litu, w wyniku której powstaje dwutlenek węgla i woda. Powstały materiał jest suszony, oczyszczany i podgrzewany w celu utworzenia bezwodnego produktu.
Zastosowanie
Ponieważ azotan litu jest rozpuszczalny w wodzie, naukowcy wykorzystują go również do syntezy związków litu przy użyciu wielu metod chemicznych opartych na roztworach. Na przykład, spalanie indukowane mikrofalami przy użyciu roztworów azotanu litu dało fosforan litowo-żelazowy typu oliwinowego (LiFePO4), tlenek litowo-kobaltowy (LiCoO2) i tlenki litowo-tytanowe (np. Li4Ti5O12 i Li2TiO3). Przetwarzanie hydrotermalne, przetwarzanie zol-żel, piroliza natryskowa, wstępne przetwarzanie współstrącania i metody suszenia emulsyjnego Li wykorzystują azotan litu jako reagent do tworzenia tlenków litu. Techniki te mogą zapewnić kontrolowaną wielkość cząstek, wielkość ziarna, krystaliczność lub ułatwić wprowadzanie domieszek w celu inżynierii właściwości produktów, często badanych pod kątem akumulatorów litowo-jonowych nowej generacji.
Nasz azotan litu klasy akumulatorowej o czystości ≥99,9% metali śladowych i niskiej zawartości zanieczyszczeń chlorkowych i siarczanowych został zaprojektowany jako prekursor materiałów katodowych do akumulatorów litowo-jonowych.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 3
Kod klasy składowania
5.1B - Oxidizing hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej