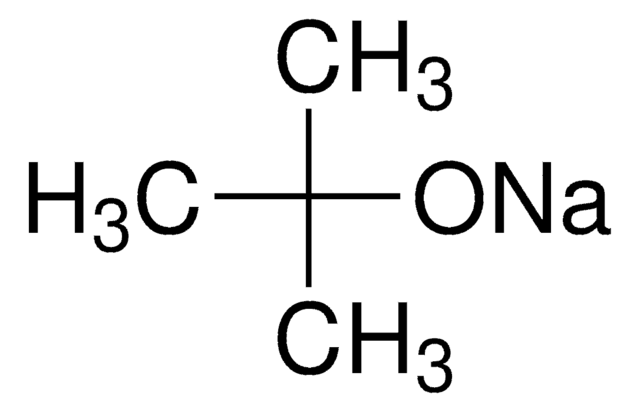
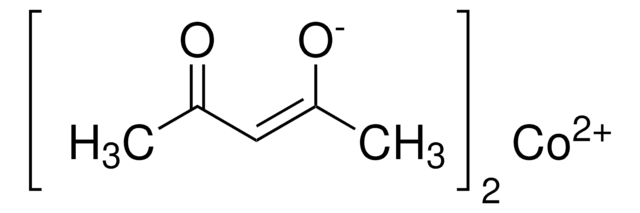
Key Documents
749125
Cobalt(II) oxo pivalate
Synonim(y):
Basic cobalt(II) pivalate, Cobalt, hexakis[μ-(2,2-dimethylpropanoato-κO:κO′)]hexakis[μ3-(2,2-dimethylpropanoato-κO:κO:κO′)]di-μ4-oxoocta-, Tetrameric Co cluster
About This Item
Polecane produkty
Postać
powder
charakterystyka ekologicznej alternatywy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
kategoria ekologicznej alternatywy
ciąg SMILES
CC(C)(C)C(=O)O[Co-]1O[Co-](O\C(=[O+]/[Co-](OC(=O)C(C)(C)C)O\C(=[O+]/[Co-](OC(=O)C(C)(C)C)OC(=O)C(C)(C)C)C(C)(C)C)C(C)(C)C)[O+]=C(O[Co-]2O[Co-](O\C(=[O+]\1)C(C)(C)C)\[O+]=C(\O[Co-](OC(=O)C(C)(C)C)\[O+]=C(\O[Co]OC(=[O+]2)C(C)(C)C)C(C)(C)C)C(C)(C)C)C(C)(C)C
InChI
1S/12C5H10O2.8Co.2O/c12*1-5(2,3)4(6)7;;;;;;;;;;/h12*1-3H3,(H,6,7);;;;;;;;;;/q;;;;;;;;;;;;4*+1;4*+2;;/p-12
Klucz InChI
PBKALLJUIAKYSI-UHFFFAOYSA-B
Opis ogólny
Zastosowanie
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej