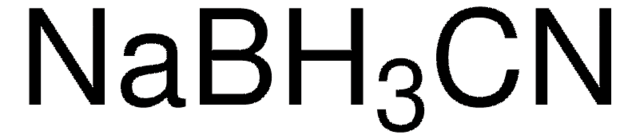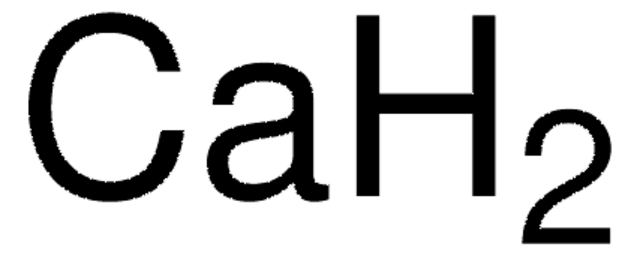Kluczowe dokumenty
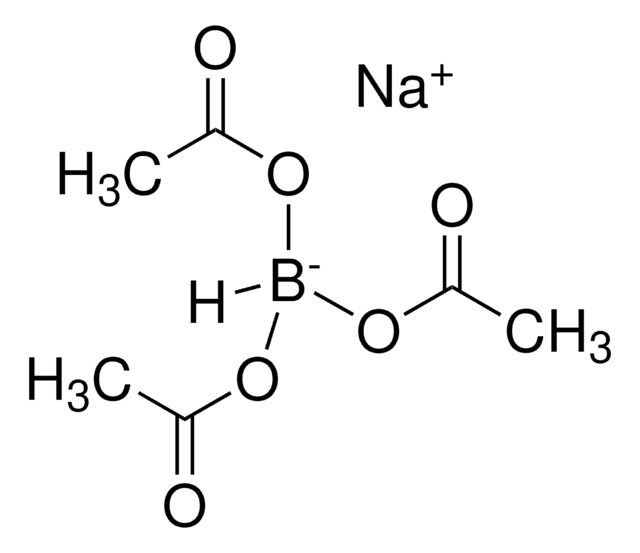
683043
Magnesium hydride
hydrogen-storage grade
About This Item
Polecane produkty
klasa czystości
hydrogen-storage grade
przydatność reakcji
reagent type: reductant
gęstość
1.45 g/mL at 25 °C (lit.)
ciąg SMILES
[Mg]
InChI
1S/Mg.2H
Klucz InChI
RSHAOIXHUHAZPM-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2 - Water-react 1
Kod klasy składowania
4.3 - Hazardous materials which set free flammable gases upon contact with water
Klasa zagrożenia wodnego (WGK)
nwg
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
How does the storage temperature relate to shipping conditions?
The storage conditions that a Sigma-Aldrich catalog and label recommend for products are deliberately conservative. For many products, long-term storage at low temperatures will increase the time during which they are expected to remain in specification and therefore are labeled accordingly. Where short-term storage, shipping time frame, or exposure to conditions other than those recommended for long-term storage will not affect product quality, Sigma-Aldrich will ship at ambient temperature. The products sensitive to short-term exposure to conditions other than their recommended long-term storage are shipped on wet or dry ice. Ambient temperature shipping helps to control shipping costs for our customers. At any time, our customers can request wet- or dry-ice shipment, but the special handling is at customer expense if our product history indicates that the product is stable for regular shipment. See Shipping and Storage for more information.
Which document(s) contains shelf-life or expiration date information for a given product?
If available for a given product, the recommended re-test date or the expiration date can be found on the Certificate of Analysis.
How do I get lot-specific information or a Certificate of Analysis?
The lot specific COA document can be found by entering the lot number above under the "Documents" section.
How do I find price and availability?
There are several ways to find pricing and availability for our products. Once you log onto our website, you will find the price and availability displayed on the product detail page. You can contact any of our Customer Sales and Service offices to receive a quote. USA customers: 1-800-325-3010 or view local office numbers.
What is the Department of Transportation shipping information for this product?
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
What is the average particle size of Product 683043, Magnesium hydride?
According to our supplier, the average particle size is approximately 50 microns.
What is the bulk density for Product 683043, Magnesium hydride?
According to our supplier, the bulk density is approximately 0.5 g/cm³.
At what temperature will magnesium hydride start to decompose?
Magnesium hydride has a decomposition temperature of 284°C.
My question is not addressed here, how can I contact Technical Service for assistance?
Ask a Scientist here.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej