Wszystkie zdjęcia(1)
Kluczowe dokumenty
433101
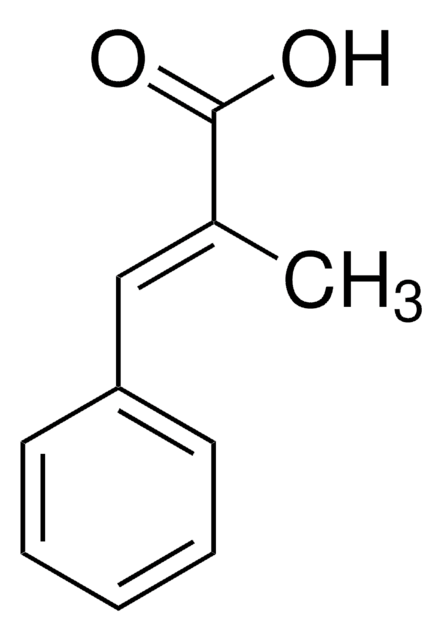
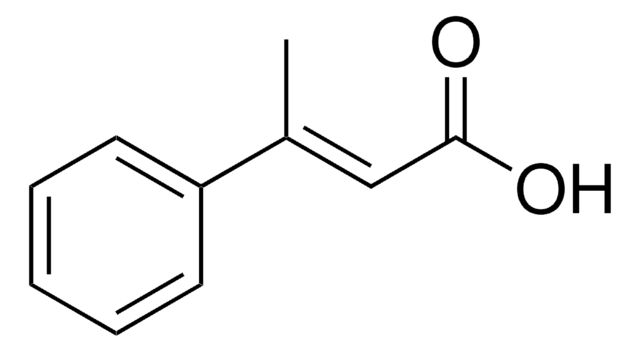
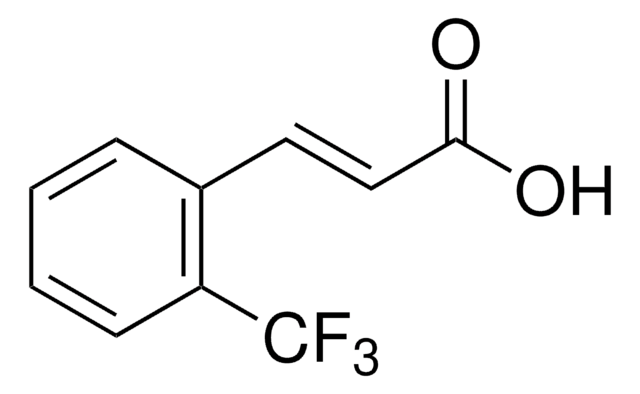
2-Methylcinnamic acid, predominantly trans
99%
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór liniowy:
CH3C6H4CH=CHCO2H
Numer CAS:
Masa cząsteczkowa:
162.19
Numer WE:
Numer MDL:
Kod UNSPSC:
12352100
Identyfikator substancji w PubChem:
NACRES:
NA.22
Polecane produkty
Poziom jakości
Próba
99%
mp
174-176 °C (lit.)
grupa funkcyjna
carboxylic acid
ciąg SMILES
Cc1ccccc1\C=C\C(O)=O
InChI
1S/C10H10O2/c1-8-4-2-3-5-9(8)6-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-6+
Klucz InChI
RSWBWHPZXKLUEX-VOTSOKGWSA-N
Opis ogólny
2-Methylcinnamic acid has been reported to exhibit strong anti-fungal activity against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus. Hydrogenation of 2-methylcinnamic acid using Walphos ligands and their biferrocene analogs has been studied.
Zastosowanie
2-Methylcinnamic acid may be used as starting reagent for the total synthesis of the cytotoxic alkaloid, 22-hydroxyacuminatine and for the preparation of (E)-2-methylcinnamic acid i-butylammonium salt.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Xiangshu Xiao et al.
Journal of medicinal chemistry, 49(4), 1408-1412 (2006-02-17)
A total synthesis of 22-hydroxyacuminatine, a cytotoxic alkaloid isolated from Camptotheca acuminata, is reported. The key step in the synthesis involves the reaction of 2,3-dihydro-1H-pyrrolo[3,4-b]quinoline with a brominated phthalide to generate a substituted pentacyclic 12H-5,11a-diazadibenzo[b,h]fluoren-11-one intermediate. Despite its structural resemblance
Sen-Sung Cheng et al.
Bioresource technology, 99(11), 5145-5149 (2007-10-20)
In this study, the antifungal activities of cinnamaldehyde and eugenol congeners against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus were evaluated and the relationships between the antifungal activity and the chemical structures were also examined. Results from antifungal
Martin E Fox et al.
The Journal of organic chemistry, 73(3), 775-784 (2007-10-24)
Four chiral diphosphine ligands consisting of bis(2,5-diphenylphospholan-1-yl) groups connected by the sp(2) carbon linkers 2,3-quinoxaline ((S,S)-Ph-Quinox), 2,3-pyrazine ((S,S)-Ph-Pyrazine), maleic anhydride ((S,S)-Ph-MalPhos), and 1,1'-ferrocene ((S,S)-Ph-5-Fc) were synthesized, and their cationic [rhodium(I)(COD)] complexes were prepared. These complexes were tested in asymmetric hydrogenation
Afrooz Zirakzadeh et al.
Organometallics, 33(8), 1945-1952 (2014-05-06)
Two representative Walphos analogues with an achiral 2,2″-biferrocenediyl backbone were synthesized. These diphosphine ligands were tested in the rhodium-catalyzed asymmetric hydrogenation of several alkenes and in the ruthenium-catalyzed hydrogenation of two ketones. The results were compared with those previously obtained
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej