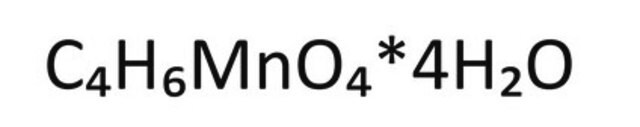Wszystkie zdjęcia(3)
Kluczowe dokumenty
330825
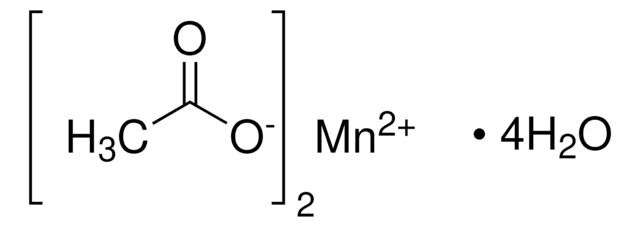
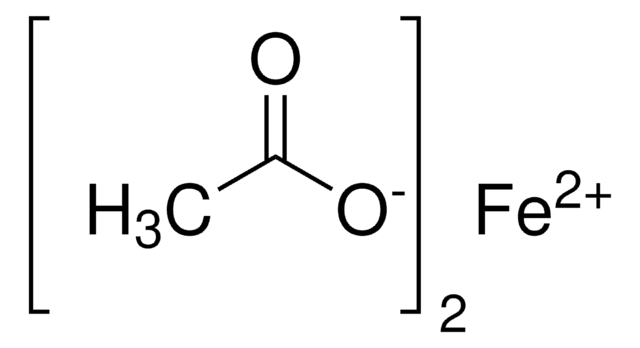
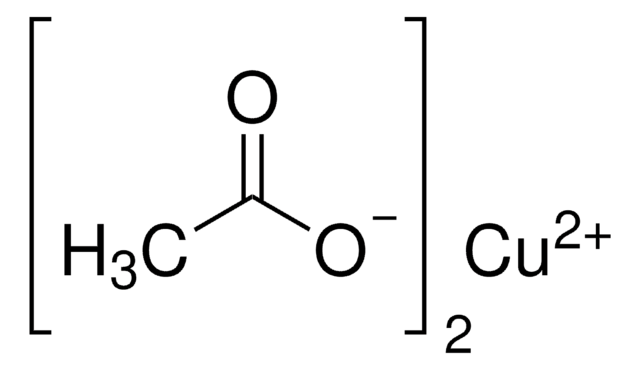
Manganese(II) acetate
98%
Synonim(y):
Diacetylomangan, Octan manganu
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór liniowy:
(CH3CO2)2Mn
Numer CAS:
Masa cząsteczkowa:
173.03
Numer WE:
Numer MDL:
Kod UNSPSC:
12352103
Identyfikator substancji w PubChem:
NACRES:
NA.23
Polecane produkty
Próba
98%
Formularz
powder
przydatność reakcji
core: manganese
ciąg SMILES
CC(=O)O[Mn]OC(C)=O
InChI
1S/2C2H4O2.Mn/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
Klucz InChI
UOGMEBQRZBEZQT-UHFFFAOYSA-L
Opis ogólny
Manganese (II) acetate is a crystalline solid that can be synthesized by reacting acetic acid withmanganese (II,III) oxide or manganese(II) carbonate. It is used as a sol-gel precursor to synthesize thin films and a critical precursor for the synthesis of cathode-active materials for rechargeable batteries.
Zastosowanie
Manganese(II) acetate can be used:
- As a precursor to synthesize manganese oxide nanoparticles for various applications such as gas sensing using the solvent-free method.
- To fabricate electrodes for high-performance Li-ion batteries.
- Suitable for the fabrication of spinel/layered heterostructured cathode materials for high-capacity and high-rate Li-Ion batteries.
- As a starting material to prepare Mn-based catalysts.
- To fabricate pyridine manganese halide scintillators for X-ray imaging.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Aquatic Chronic 3 - STOT RE 2 Inhalation
Organy docelowe
Brain
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Eleonora Pargoletti et al.
Nanomaterials (Basel, Switzerland), 10(9) (2020-09-05)
One of the major drawbacks in Lithium-air batteries is the sluggish kinetics of the oxygen reduction reaction (ORR). In this context, better performances can be achieved by adopting a suitable electrocatalyst, such as MnO2. Herein, we tried to design nano-MnO2
Tingwei Zhang et al.
ChemSusChem, 11(16), 2730-2736 (2018-06-01)
The rational design of highly efficient and durable oxygen reduction reaction (ORR) catalysts is critical for the commercial application of fuel cells. Herein, three-dimensional graphene (3D-G) is synthesized by the template method, which used coal tar pitch as the carbon
Fangchun Han et al.
Chemistry, an Asian journal, 12(17), 2284-2290 (2017-08-02)
This work demonstrates a facile in situ synthesis of cobalt-manganese mixed sulfide (CoMn-S) nanocages on reduced graphene oxide (RGO) sheets by using a crystalline Co-Mn precursor as the sacrificial template. The CoMn-S/RGO hybrid was applied as the anode for Li-ion
Eesh Vaghela et al.
Physical chemistry chemical physics : PCCP, 19(7), 5163-5176 (2017-02-01)
In this communication, structural, microstructural, transport and magnetotransport properties are reported for La
Jia Yao et al.
Chemical science, 9(11), 2927-2933 (2018-05-08)
Reactive oxygen species (ROS)-induced oxidative stress is linked to various diseases, including cardiovascular disease and cancer. Though highly efficient natural ROS scavenging enzymes have been evolved, they are sensitive to environmental conditions and hard to mass-produce. Therefore, enormous efforts have
Produkty
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej