Wszystkie zdjęcia(1)
Key Documents
19240
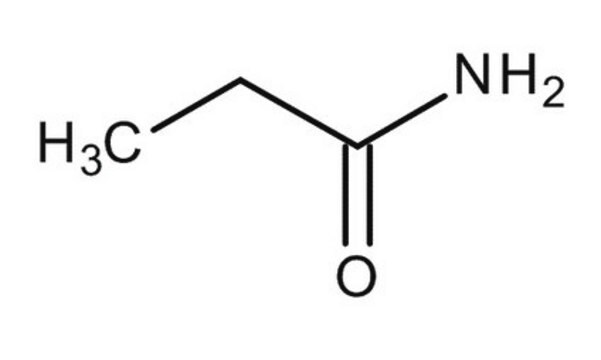
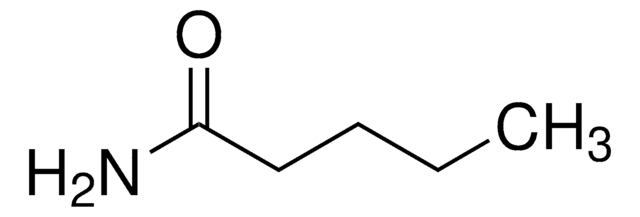
Butyramide
≥98.0% (T)
Synonim(y):
Amide C4
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór liniowy:
CH3CH2CH2CONH2
Numer CAS:
Masa cząsteczkowa:
87.12
Beilstein:
1361528
Numer WE:
Numer MDL:
Kod UNSPSC:
12352100
Identyfikator substancji w PubChem:
NACRES:
NA.22
Polecane produkty
Poziom jakości
Próba
≥98.0% (T)
mp
114-116 °C
rozpuszczalność
alcohol: soluble(lit.)
diethyl ether: slightly soluble(lit.)
water: soluble(lit.)
grupa funkcyjna
amide
ciąg SMILES
CCCC(N)=O
InChI
1S/C4H9NO/c1-2-3-4(5)6/h2-3H2,1H3,(H2,5,6)
Klucz InChI
DNSISZSEWVHGLH-UHFFFAOYSA-N
informacje o genach
rat ... Ggt1(116568)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
Butyramide was used in the synthesis of hydroxamic acids, electrorheological fluids and β-amodoorganotin compounds. It was used as substrate of (+)-γ-lactamase to develop a microreactor to study enzyme stability, activity, kinetics and substrate specificity.
This page may contain text that has been machine translated.
Kod klasy składowania
13 - Non Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Hongjian Zhang et al.
Drug metabolism and disposition: the biological fate of chemicals, 35(5), 795-805 (2007-02-17)
2-{Butyryl-[2'-(4,5-dimethyl-isoxazol-3-ylsulfamoyl)-biphenyl-4-ylmethyl]-amino}-N-isopropyl-3-methyl-butyramide (BMS-1) is a potent dual acting angiotensin-1 and endothelin-A receptor antagonist. The compound was subject to rapid metabolic clearance in monkey and human liver microsomes and exhibited low systemic exposure and marked interanimal variability in cynomolgus monkeys after p.o.
Effect of butyrate analogues on proliferation and differentiation in human neuroblastoma cell lines.
P Rocchi et al.
Anticancer research, 18(2A), 1099-1103 (1998-06-06)
Butyric acid has been shown in vitro to produce cytodifferentiation of a wide variety of neoplastic cells. The potential clinical use of this compound as a therapeutic agent is limited by its rapid metabolism. This has led to the examination
B P O'Hara et al.
Protein engineering, 13(2), 129-132 (2000-03-10)
The AmiC protein in Pseudomonas aeruginosa is the negative regulator and ligand receptor for an amide-inducible aliphatic amidase operon. In the wild-type PAC1 strain, amidase expression is induced by acetamide or lactamide, but not by butyramide. A mutant strain of
Rajendra Singh et al.
Bioprocess and biosystems engineering, 41(8), 1225-1232 (2018-05-12)
Butyramide is a commodity chemical having wide range of applications from material science to biological sciences including synthesis of therapeutic drugs, hydroxamic acids, and electrorheological fluids. The nitrile hydratase protein of Bacillus sp. APB-6 was explored to develop an efficient
R A Norman et al.
The Journal of biological chemistry, 275(39), 30660-30667 (2000-07-13)
Expression of the amidase operon of Pseudomonas aeruginosa is controlled by AmiC, the ligand sensor and negative regulator, and AmiR the transcription antitermination factor activator. We have titrated out AmiC repression activity in vivo by increased AmiR production in trans
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej