754218
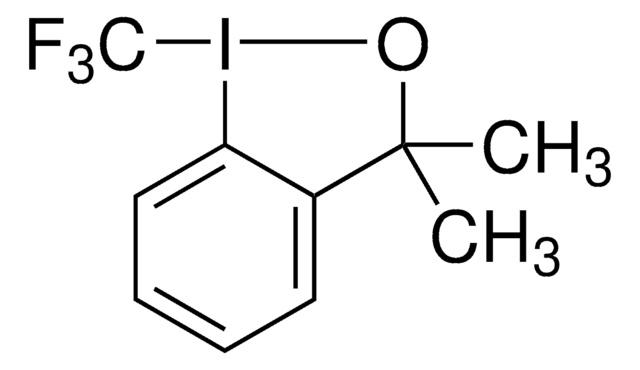
3,3-Dimethyl-1-(trifluoromethyl)-1,2-benziodoxole
98%
동의어(들):
1,3-Dihydro-3,3-dimethyl-1-(trifluoromethyl)-1,2-benziodoxole, Togni’s Reagent
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C10H10F3IO
CAS Number:
Molecular Weight:
330.09
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
양식
powder
mp
75-79 °C
저장 온도
2-8°C
SMILES string
CC1(C)O[I](c2ccccc12)C(F)(F)F
InChI
1S/C10H10F3IO/c1-9(2)7-5-3-4-6-8(7)14(15-9)10(11,12)13/h3-6H,1-2H3
InChI key
HVAPLSNCVYXFDQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Selective trifluoromethylation of 1,3-disubstituted arenes through iridium-catalyzed arene borylation and copper-catalyzed trifluoromethylation
- Copper-catalyzed trifluoromethylation of aryl- and alkenylboronic acids with electrophilic trifluoromethylating reagent
- Pd-catalyzed electrophilic ortho-trifluoromethylation of arenes using trifluoroacetic acid as a promotor
Used in the Preparation of
- Trifluoromethylimidoylethyl substituted heterocycles via bis(trifluoromethylsulfonyl)amine-catalyzed Rotter type reaction of heterocycles with nitriles in presence of trifluoromethylbenziodoxole
- Stereoselective synthesis of α-trifluoromethyl aldehydes via trimethylbenzylimidazolidinone and copper-catalyzed enantioselective α-trifluoromethylation of aldehydes with iodonium salts
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Tianfei Liu et al.
Angewandte Chemie (International ed. in English), 51(2), 540-543 (2011-12-01)
The old one two: A sequential iridium-catalyzed borylation and copper-catalyzed trifluoromethylation of arenes is described (see scheme; Pin = pinacol). The reaction is conducted under mild reaction conditions and tolerates a variety of functional groups. The advantages of this tandem
Tianfei Liu et al.
Organic letters, 13(9), 2342-2345 (2011-04-09)
A copper-catalyzed trifluoromethylation of aryl- and alkenylboronic acids with Togni's reagent was described. The reaction proceeded in good to excellent yields for a range of different substrates including heteroarylboronic acids and substrates with a variety of functional groups under mild
Anna E Allen et al.
Journal of the American Chemical Society, 132(14), 4986-4987 (2010-03-20)
An enantioselective organocatalytic alpha-trifluoromethylation of aldehydes has been accomplished using a commercially available, electrophilic trifluoromethyl source. The merging of Lewis acid and organocatalysis provides a new strategy for the enantioselective construction of trifluoromethyl stereogenicity, an important chiral synthon for pharmaceutical
Xisheng Wang et al.
Journal of the American Chemical Society, 132(11), 3648-3649 (2010-02-27)
A Pd(II)-catalyzed C-H activation/trifluoromethylation of arenes with an electrophilic trifluoromethylation reagent using diverse heterocycle directing groups is reported. The presence of trifluoroacetic acid is crucial for this catalytic reaction.
A Ritter-type reaction: direct electrophilic trifluoromethylation at nitrogen atoms using hypervalent iodine reagents.
Katrin Niedermann et al.
Angewandte Chemie (International ed. in English), 50(5), 1059-1063 (2011-01-27)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.