모든 사진(1)
About This Item
실험식(Hill 표기법):
C9H21N3
CAS Number:
Molecular Weight:
171.28
Beilstein:
2352408
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥97.0% (GC)
형태
liquid
refractive index
n20/D 1.457
bp
88-89 °C/43 mmHg (lit.)
SMILES string
CN(C)\C(=N/C(C)(C)C)N(C)C
InChI
1S/C9H21N3/c1-9(2,3)10-8(11(4)5)12(6)7/h1-7H3
InChI key
YQHJFPFNGVDEDT-UHFFFAOYSA-N
일반 설명
2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton′s base) is an excellent alternative to traditional inorganic bases for promoting the coupling reaction.
애플리케이션
- Synthesis of dinaphthyl ethers: Barton′s base, which includes 2-tert-Butyl-1,1,3,3-tetramethylguanidine, was utilized to promote SNAr reactions for the synthesis of highly oxygenated dinaphthyl ethers, demonstrating its efficacy as a catalyst in complex organic synthesis processes (Wipf and Lynch, 2003).
주의사항
Remark on appearance: Material may form precipitate on storage. The precipitate may easily be separated by filtration.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
149.0 °F - closed cup
Flash Point (°C)
65.0 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
D.H.R. Barton et al.
Organic Syntheses, 74, 103-103 (1997)
Peter Wipf et al.
Organic letters, 5(7), 1155-1158 (2003-03-28)
[reaction: see text] Electron-rich dinaphthyl ethers were synthesized by S(N)Ar reactions between naphthols and activated fluoronaphthalenes. 2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton's base) was found to be an excellent, mild alternative to traditional inorganic bases for promoting the coupling reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.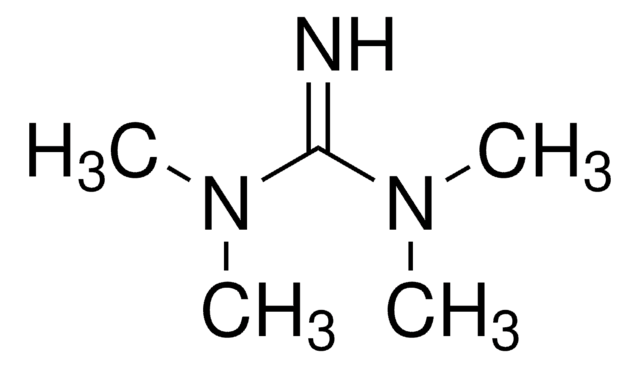
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)
![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)



![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
![1,5-Diazabicyclo[4.3.0]non-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/400/401/859b2474-712b-4448-b231-74d0bc3203f1/640/859b2474-712b-4448-b231-74d0bc3203f1.png)