추천 제품
vapor pressure
5.3 mmHg ( 37.7 °C)
Quality Level
분석
98%
형태
liquid
환경친화적 대안 제품 특성
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.522-1.524 (lit.)
bp
80-83 °C/0.6 mmHg (lit.)
density
1.018 g/mL at 25 °C (lit.)
환경친화적 대안 카테고리
, Aligned
SMILES string
C1CCN2CCCN=C2CC1
InChI
1S/C9H16N2/c1-2-5-9-10-6-4-8-11(9)7-3-1/h1-8H2
InChI key
GQHTUMJGOHRCHB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- as catalyst for carboxylic acid esterification with dimethyl carbonate
- in the synthesis of duocarmycin and CC-1065 analogs
- as catalyst in aza-Michael addition and Knovenegal condensation reaction
- as base for dehalogenation of halogenated Diels-Alder adducts and the resulting activated 2,4-dienones were subjected to regio- and stereo-directed Michael additions, using Yamamoto′s reagent (CH3Cu · BF3)
- in a new synthesis of the ABCD ring system of Camptothecin
특징 및 장점
문헌인용
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point (°F)
240.8 °F
Flash Point (°C)
116 °C
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
문서
The prevalence of organofluorine compounds in industry and drug design necessitates the ability to introduce C–F bonds to molecules.
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
A solid and bench-stable alternative to sulfuryl fluoride gas has been developed, 4-(Acetylamino)phenyl]imidodisulfuryl difluoride (ASIF). ASIF is a shelf-stable, crystallilne reagent for the installation of the valuable SO2F functional group.
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![1,8-Diazabicyclo[5.4.0]undec-7-ene puriss., ≥99.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene for synthesis](/deepweb/assets/sigmaaldrich/product/images/219/652/f12d7266-2d82-4869-9d8d-919b0f68de68/640/f12d7266-2d82-4869-9d8d-919b0f68de68.jpg)
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
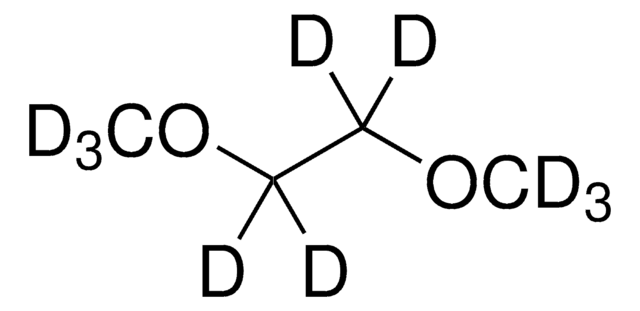

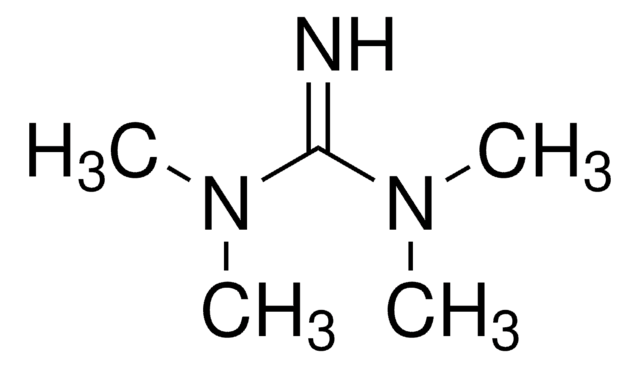

![1,5-Diazabicyclo[4.3.0]non-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/400/401/859b2474-712b-4448-b231-74d0bc3203f1/640/859b2474-712b-4448-b231-74d0bc3203f1.png)


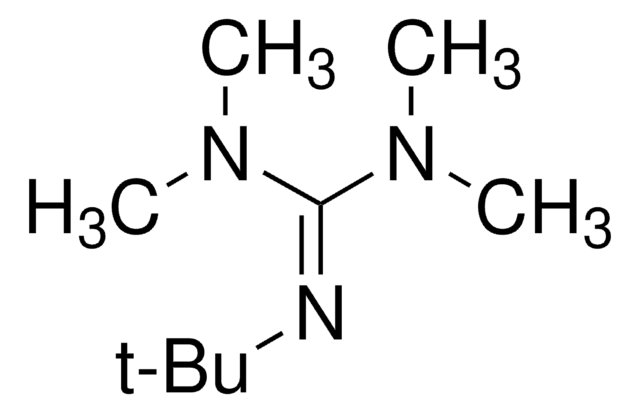
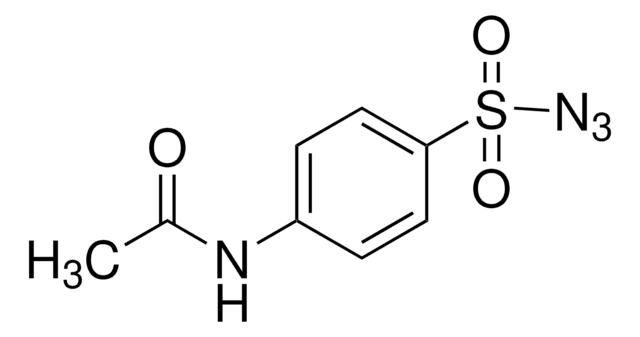
![3,3,6,9,9-Pentamethyl-2,10-diazabicyclo[4.4.0]dec-1-ene ≥96.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/203/819/340f3f5a-eaa1-4393-8425-631460e3154d/640/340f3f5a-eaa1-4393-8425-631460e3154d.png)