11625
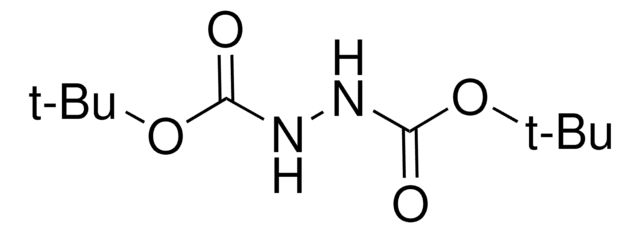
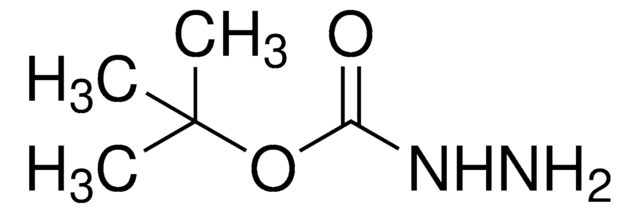
Di-tert-butyl azodicarboxylate
purum, ≥98.0% (GC)
동의어(들):
Bis(1,1-dimethylethyl)azodicarboxylate, DBAD, Di-tert-butyl azodiformate, NSC 109889
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
(CH3)3COCON=NCOOC(CH3)3
CAS Number:
Molecular Weight:
230.26
Beilstein:
1911434
EC Number:
MDL number:
UNSPSC 코드:
12352108
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Di-tert-butyl azodicarboxylate is a an acid labile reagent for Mitsunobu reactions allowing facile isolation of products and for the electrophilic amination and hydrazination of enolates and lithium alkyls.
애플리케이션
Reactant for:
- Preparation of hexapeptide key fragments via stereoselective selenocyclization/oxidative deselenylation or hydrazination/cyclization reactions
- Asymmetric Michael addition reactions
- Preparation of dipeptidyl peptidase IV dependent water-soluble prodrugs via Mitsunobu reaction
- Synthesis of pyrroloisoquinoline template via stereoselective N-acyliminium-mediated cyclization and enolate amination for synthesis of peptidomimetic compounds
- Barbier-type propargylation reactions
- Synthesis of bacterial peptide deformylase (PDF) inhibitor fumimycin
- Asymmetric amination of glycine Schiff bases
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
C. Gennari et al.
Journal of the American Chemical Society, 108, 6394-6394 (1986)
Cong-Bin Ji et al.
Organic & biomolecular chemistry, 10(6), 1158-1161 (2012-01-11)
We report the first example of catalytic asymmetric direct amination of α-monosubstituted nitroacetates using di-tert-butyl azodicarboxylate. The simple and easily available Hatakeyama's catalyst β-ICD 11 was found to be a highly enantioselective catalyst for this reaction.
J.L. Vicario et al.
Tetrahedron Letters, 40, 7123 -7123 (1999)
D.A. Evans et al.
Journal of the American Chemical Society, 108, 6395-6395 (1986)
M. Kiankarimi et al.
Tetrahedron Letters, 40, 4497-4497 (1999)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.