902489
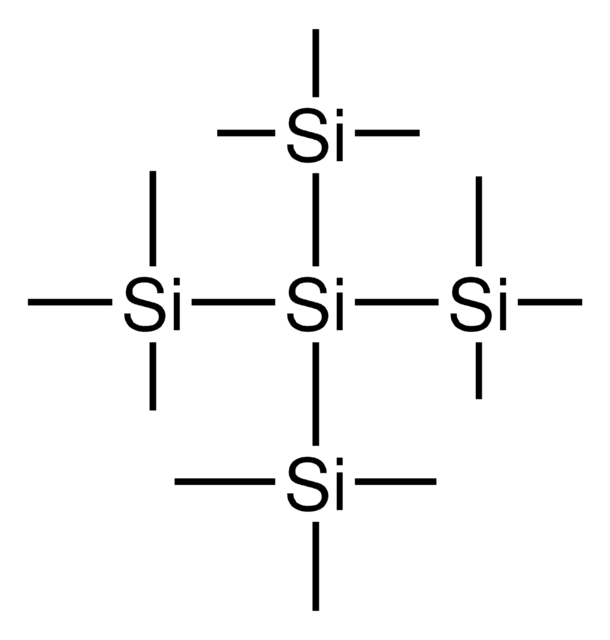
Tris(trimethylsilyl)silanol
≥95%
동의어(들):
(Hydroxy-bis(trimethylsilyl)silyl)-trimethylsilane, (TMS)3SiOH, 1,1,1,3,3,3-Hexamethyl-2-(trimethylsilyl)trisilan-2-ol, Supersilanol
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C9H28OSi4
CAS Number:
Molecular Weight:
264.66
MDL number:
UNSPSC 코드:
12161700
NACRES:
NA.22
추천 제품
분석
≥95%
양식
liquid
반응 적합성
reaction type: C-C Bond Formation
refractive index
n/D 1.496
density
0.859 g/mL
SMILES string
[Si]([Si](C)(C)C)([Si](C)(C)C)([Si](C)(C)C)O
InChI
1S/C9H28OSi4/c1-11(2,3)14(10,12(4,5)6)13(7,8)9/h10H,1-9H3
InChI key
ABTWCNHNRLMBFR-UHFFFAOYSA-N
애플리케이션
Under a dual catalytic copper/photoredox manifold, this supersilanol has been demonstrated by the MacMillan lab to be an excellent reagent for the trifluoromethylation of alkyl halides and aryl halides to yield alkyl-CF3 and aryl-CF3 in high yields. In both cases, these reactions exhibit wide substrate scope with good functional group tolerance. More specifically, a variety of 5-membered and 6-membered heteroaryl halides can be readily converted to the corresponding trifluoromethylheteroarenes under mild conditions. To be use in conjunction with dMesSCF3 (901466) and Ir photocatalyst (902217 or 902225).
관련 제품
제품 번호
설명
가격
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
200.3 °F
Flash Point (°C)
93.5 °C
이미 열람한 고객
Timothy J Boyle et al.
Inorganic chemistry, 57(15), 8806-8820 (2018-07-07)
In an effort to generate single-source precursors for the production of metal-siloxide (MSiO x) materials, the tris(trimethylsilyl)silanol (H-SST or H-OSi(SiMe3)3 (1) ligand was reacted with a series of group 4 and 5 metal alkoxides. The group 4 products were crystallographically
Annie J Jiang et al.
Journal of the American Chemical Society, 131(46), 16630-16631 (2009-11-19)
Mo and W MonoAryloxide-Pyrrolide (MAP) olefin metathesis catalysts can couple terminal olefins to give as high as >98% Z-products in moderate to high yields with as little as 0.2% catalyst. Results are reported for 1-hexene, 1-octene, allylbenzene, allyltrimethylsilane, methyl-10-undecenoate, methyl-9-decenoate
Chip Le et al.
Science (New York, N.Y.), 360(6392), 1010-1014 (2018-06-02)
Transition metal-catalyzed arene functionalization has been widely used for molecular synthesis over the past century. In this arena, copper catalysis has long been considered a privileged platform due to the propensity of high-valent copper to undergo reductive elimination with a
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)