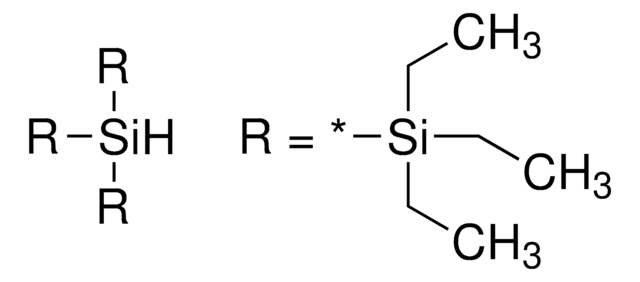
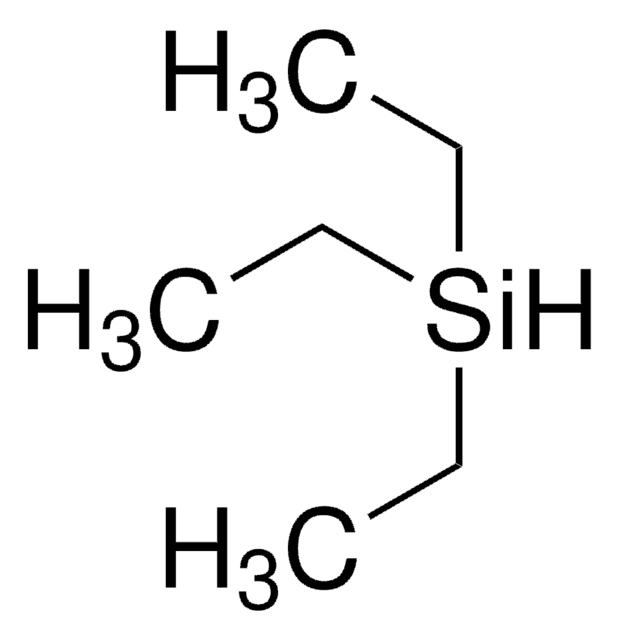
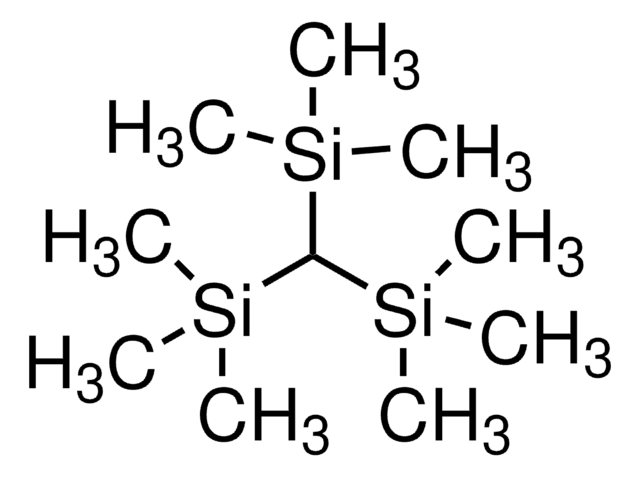
추천 제품
Quality Level
분석
97%
양식
liquid
반응 적합성
reagent type: reductant
refractive index
n20/D 1.489 (lit.)
bp
73 °C/5 mmHg (lit.)
density
0.806 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(C)[SiH]([Si](C)(C)C)[Si](C)(C)C
InChI
1S/C9H28Si4/c1-11(2,3)10(12(4,5)6)13(7,8)9/h10H,1-9H3
InChI key
SQMFULTZZQBFBM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
Used in:
- Hydrosilylations
- Radical reactions
- Reductions of acid chlorides
- Reductions of carbon-halogen bonds
- Hydrosilations of carbonyls
- Another common application involves the use of the tris(trimethylsilyl)silyl (TTMSS, or super silyl) group when complexed with transition metals and main group elements
- More recently, the super silyl group is being utilized in carbon–carbon bond forming reactions
이미 열람한 고객
문서
The super silyl group is a powerful tool for the synthetic chemist, showing great efficacy in various carbon–carbon bond forming reactions.
The super silyl group is a powerful tool for the synthetic chemist, showing great efficacy in various carbon–carbon bond forming reactions.
This article briefly reviews the methods and mechanisms for the formation of molecular monolayers on silicon surfaces, the properties of these monolayers and current perspectives regarding their application in molecular electronic and sensing applications.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.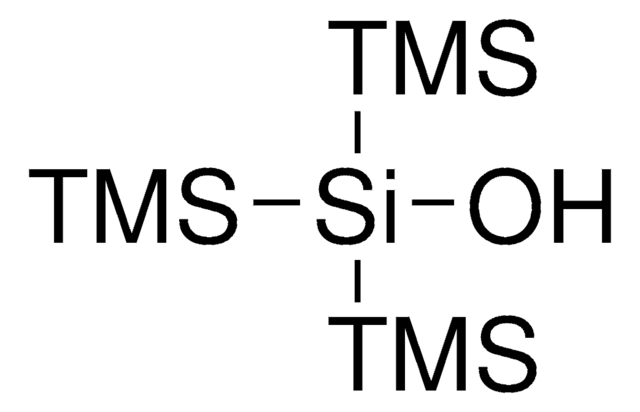
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)