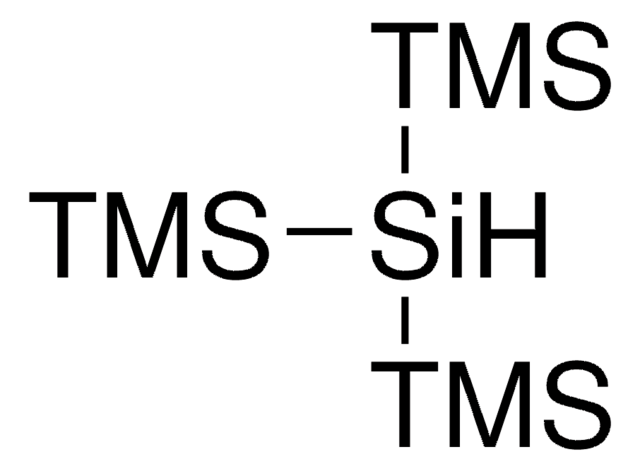736856
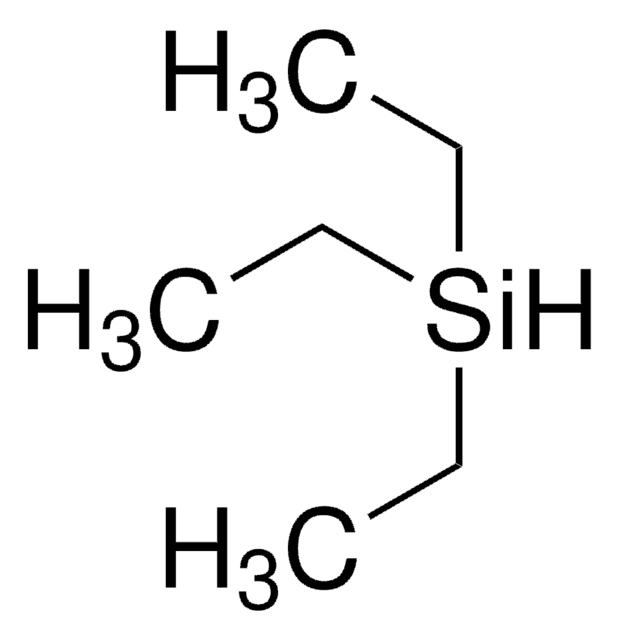
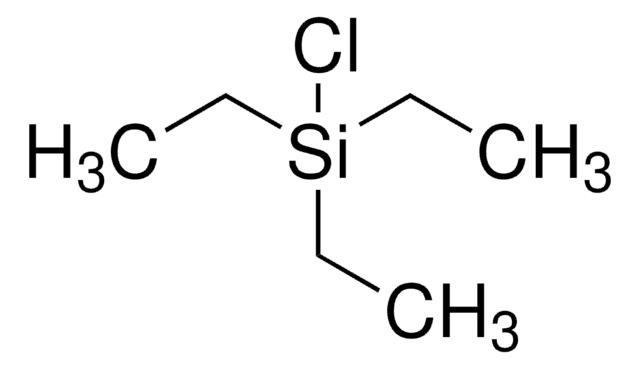
Tris(triethylsilyl)silane
동의어(들):
1,1,1,3,3,3-Hexaethyl-2-(triethylsilyl)trisilane, 3,4,5-Trisilaheptane, 3,3,5,5-tetraethyl-4-(triethylsilyl)
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C18H46Si4
CAS Number:
Molecular Weight:
374.90
MDL number:
UNSPSC 코드:
12352001
PubChem Substance ID:
NACRES:
NA.22
추천 제품
양식
liquid
Quality Level
반응 적합성
reagent type: reductant
refractive index
n20/D 1.526
density
0.887 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
CC[Si](CC)(CC)[SiH]([Si](CC)(CC)CC)[Si](CC)(CC)CC
InChI
1S/C18H46Si4/c1-10-20(11-2,12-3)19(21(13-4,14-5)15-6)22(16-7,17-8)18-9/h19H,10-18H2,1-9H3
InChI key
WNGZMQFMMHZKBG-UHFFFAOYSA-N
애플리케이션
Tris(triethylsilyl)silane can be incorporated as a directing group for various regio- and stereo-selective reactions. Hydrogen abstraction from tris(triethylsilyl)silane yields highly stable silyl radical.
Tris(triethylsilyl)silane can be used as a hydrogen atom donor reagent in chemical synthesis due to its weak Si-H bond. Hydrogen abstraction from tris(triethylsilyl)silane yields a highly stable silyl radical.
It can be used as a reagent:
It can be used as a reagent:
- In the radical coupling reaction to generate C-C bonds from alkyl-halogen compounds using iridium and nickel catalysts.
- To synthesize α-arylated product via cross-electrophile coupling reaction between α-chloro carbonyl and aryl bromide in the presence of nickel and iridium catalysts.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Synthesis of ?-Hydroxy-?-haloesters through Super Silyl Ester Directed Syn-Selective Aldol Reaction.
Oda S and Yamamoto H
Organic Letters, 15(23), 6030-6033 (2013)
Selective Michael Reaction Controlled by Supersilyl Protecting Group.
Izumiseki A and Yamamoto H
Angewandte Chemie (International Edition in English), 127(30), 8821-8823 (2015)
Generation of Organolithium Compounds bearing Super Silyl Ester and their Application to Matteson Rearrangement.
Oda S and Yamamoto H
Angewandte Chemie (International Edition in English), 125(31), 8323-8326 (2013)
Controlling stereochemistry in polyketide synthesis: 1, 3-vs. 1, 2-asymmetric induction in methyl ketone aldol additions to ?-super siloxy aldehydes.
Brady PB, et al.
Chemical Science, 4(8), 3223-3231 (2013)
Highly Stable Silyl Radicals (Et n Me3-n Si) 3Si?(n= 1? 3).
Kyushin S, et al.
Organometallics, 16(25), 5386-5388 (1997)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.