225649
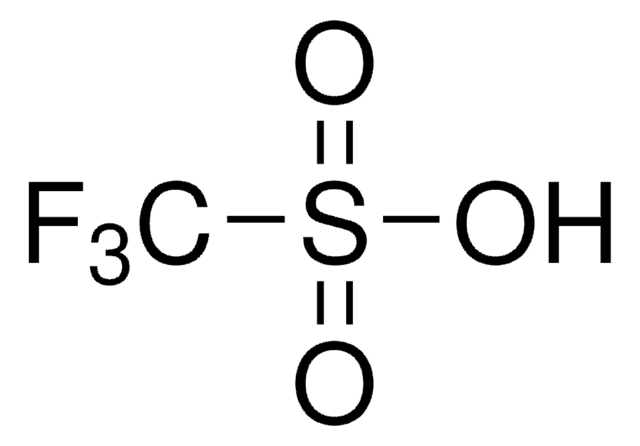
Trimethylsilyl trifluoromethanesulfonate
99%
동의어(들):
TMS triflate, TMSOTf, Trifluoromethanesulfonic acid trimethylsilylester
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CF3SO3Si(CH3)3
CAS Number:
Molecular Weight:
222.26
Beilstein:
1868911
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Trimethylsilyl trifluoromethanesulfonate has been used in combination with boron trifluoride etherate for the copper-catalyzed asymmetric allylic alkylation (AAA) of allyl bromides, chlorides, and ethers with organolithium reagents in the presence of a chiral ligand.
It can be used:
It may also be used to catalyze:
It can be used:
- As a silylating agent for the synthesis of trimethylsilyl-enol ethers from esters of α-diazoacetoacetic acid.
- To activate benzyl and allyl ethers for the alkylation of sulfides.
- To facilitate the conversion of Diels-Alder adducts of Danishefsky′s diene to cyclohexenones without the formation of methoxy ketone by-product.
- To prepare difluoroboron triflate etherate, a powerful Lewis acid especially in acetonitrile solvent.
- As a reagent in a Dieckmann-like cyclization of ester-imides and diesters.
It may also be used to catalyze:
- Acylation of alcohols with acid anhydrides.
- Reductive coupling of carbonyl compounds with trialkylsilanes to form symmetrical ethers.
- Glycosidation of 4-demethoxydaunomycinones with 1-O-acyl-L-daunosamine derivatives.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
77.0 °F - closed cup
Flash Point (°C)
25 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
A simple method of preparing trimethylsilyl-and tert-butyldimethylsilyl-enol ethers of ?-diazoacetoacetates and their use in the synthesis of a chiral precursor to thienamycin analogs.
Ueda Y, et al.
Canadian Journal of Chemistry, 62(12), 2936-2940 (1984)
Thomas R Hoye et al.
Organic letters, 8(23), 5191-5194 (2006-11-03)
[Structure: see text] Trialkylsilyl triflates effect cyclization of ester-imides such as 2 to produce adducts such as 4a. Trapping of the in situ generated, nucleophilic ketene acetal (cf. 5a) is a key aspect of the transformation. A range of substrates
BF 3? OEt 2 and TMSOTf: A synergistic combination of Lewis acids.
Myers EL, et al.
Chemical Communications (Cambridge, England), 42, 4434-4436 (2006)
Eddie L Myers et al.
Chemical communications (Cambridge, England), (42), 4434-4436 (2006-10-24)
The combination of BF3.OEt2 and TMSOTf gives BF2OTf.OEt2, which is a more powerful Lewis acid than its components and especially effective in CH3CN solvent; the complex formed has been characterised by 1H, 19F, 11B and 31P (using Et3PO as an
Method for sulfide S-benzylation or S-allylation using trimethylsilyl triflate activated benzyl or allyl ethers.
Vedejs E & Eustache J.
The Journal of Organic Chemistry, 46(16), 3353-3354 (1981)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.